纠缠三重对态的相干光激发
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
有机半导体的功能特性是由光学亮态和暗态之间的相互作用决定的。有机器件需要在这些明暗流形之间快速转换,以实现最高效率,而实现这一目标的方法之一就是多激子生成(S1→1TT)。暗态 1TT 通常是在光激发后由亮态 S1 生成的;然而,关于其机理细节还存在着激烈的争论。在这里,我们报告了一种 1TT 的生成途径,在这种途径中,1TT 可以被相干光激发,而不需要亮 S1 的参与。利用 10 fs 瞬态吸收光谱和亚共振泵浦,1TT 可以直接从基态产生。我们将这种方法应用于一系列并五苯二聚物和各种聚集类型的薄膜,从而确定了实现这种禁止途径的关键材料特性。这项成果通过一种极其简单的技术,为从机理上深入了解有机材料中的 1TT 和其他暗态打开了一扇大门。本文章由计算机程序翻译,如有差异,请以英文原文为准。
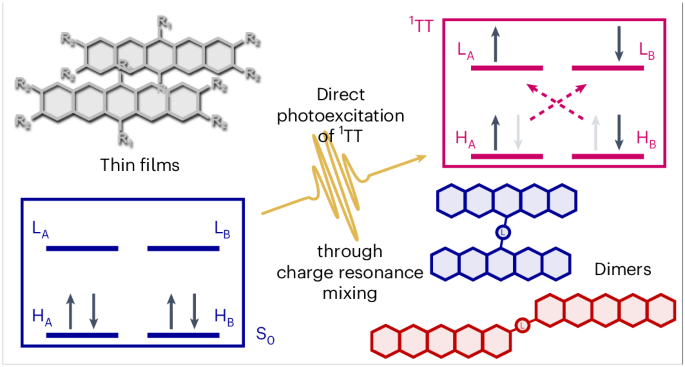
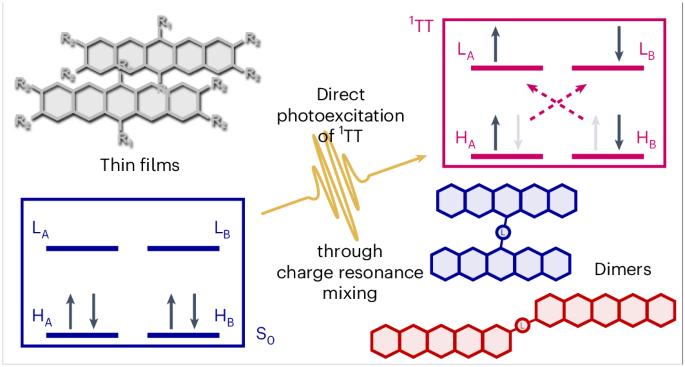
Coherent photoexcitation of entangled triplet pair states
The functional properties of organic semiconductors are defined by the interplay between optically bright and dark states. Organic devices require rapid conversion between these bright and dark manifolds for maximum efficiency, and one way to achieve this is through multiexciton generation (S1→1TT). The dark state 1TT is typically generated from bright S1 after optical excitation; however, the mechanistic details are hotly debated. Here we report a 1TT generation pathway in which it can be coherently photoexcited, without any involvement of bright S1. Using <10-fs transient absorption spectroscopy and pumping sub-resonantly, 1TT is directly generated from the ground state. Applying this method to a range of pentacene dimers and thin films of various aggregation types, we determine the critical material properties that enable this forbidden pathway. Through a strikingly simple technique, this result opens the door for new mechanistic insights into 1TT and other dark states in organic materials. The mechanistic details of entangled triplet pair formation in organic materials have been debated over the past decade. Now, the concept of coherent triplet pair formation is revived using a library of pentacene derivatives, invoking charge resonance mixing as a material design principle for harnessing the effect.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


