3',6'-二取代的光谱霉素的合成和抗菌作用。
IF 2.1
4区 医学
Q3 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
光谱霉素是一种氨基环醇抗生素,具有独特的核糖体结合位点。之前对光谱霉素进行合成修饰时,通过在 6'-位上添加光谱霉素以生成特罗霉素,在 3'-位上添加光谱酰胺以生成光谱霉素和氨甲基光谱霉素,从而增强了光谱霉素的效力和抗菌谱。本研究的重点是设计、合成和评估三种 3',6'-二取代的光谱霉素类似物:Trospectinamide、N-苄基连接的氨甲基和 N-乙烯连接的氨甲基 trospectomycins。计算实验预测,这些二取代类似物能够与 SPC 核糖体结合位点结合。这些新的类似物是根据之前确立的光谱酰胺和氨甲基光谱霉素系列的合成路线,从rospectomycin 合成的。在无细胞翻译试验中,二取代类似物显示出与光谱霉素或曲博霉素相似的核糖体抑制作用。这些二取代类似物对多种细菌具有抑制 MIC 活性,3'-修饰决定了其活性谱,从而提高了对分枝杆菌的活性。值得注意的是,N-乙烯连接的氨甲基曲安奈德对脓肿分枝杆菌的效力增强,曲安奈德对结核杆菌的活性也很强,这与光谱酰胺类药物的选择性功效一致。研究还发现,在结核分枝杆菌和脓肿分枝杆菌中,曲螺霉素容易外流。这些发现有助于了解光谱霉素类似物的结构-活性关系,并可指导设计和合成更有效的光谱霉素化合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。

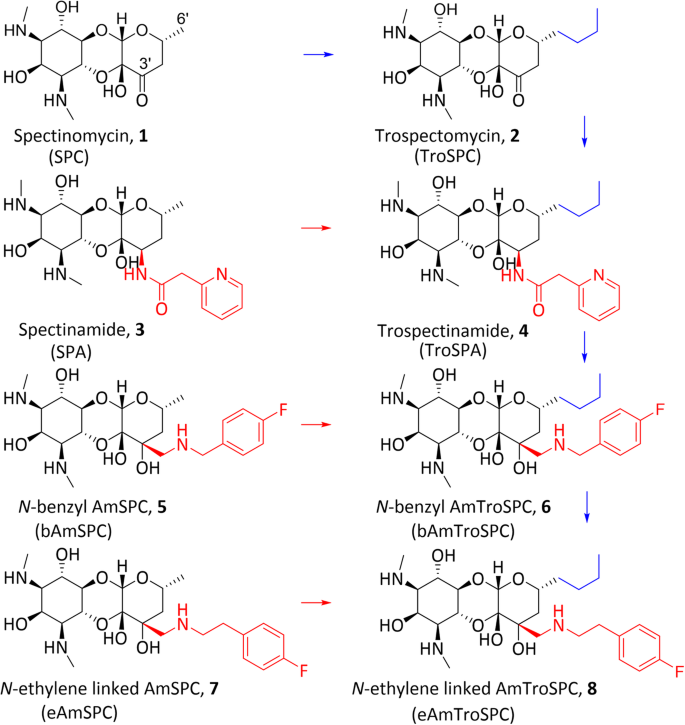
Synthesis and antibacterial action of 3’,6’-disubstituted spectinomycins
Spectinomycin is an aminocyclitol antibiotic with a unique ribosomal binding site. Prior synthetic modifications of spectinomycin have enhanced potency and antibacterial spectrum through addition at the 6’-position to produce trospectomycin and to the 3’-position to produce spectinamides and aminomethyl spectinomycins. This study focused on the design, synthesis, and evaluation of three 3’,6’-disubstituted spectinomycin analogs: trospectinamide, N-benzyl linked aminomethyl, and N-ethylene linked aminomethyl trospectomycins. Computational experiments predicted that these disubstituted analogs would be capable of binding within the SPC ribosomal binding site. The new analogs were synthesized from trospectomycin, adapting the previously established routes for the spectinamide and aminomethyl spectinomycin series. In a cell-free translation assay, the disubstituted analogs showed ribosomal inhibition similar to spectinomycin or trospectomycin. These disubstituted analogs demonstrated inhibitory MIC activity against various bacterial species with the 3’-modification dictating spectrum of activity, leading to improved activity against mycobacterium species. Notably, N-ethylene linked aminomethyl trospectomycins exhibited increased potency against Mycobacterium abscessus and trospectinamide displayed robust activity against M. tuberculosis, aligning with the selective efficacy of spectinamides. The study also found that trospectomycin is susceptible to efflux in M. tuberculosis and M. abscessus. These findings contribute to the understanding of the structure-activity relationship of spectinomycin analogs and can guide the design and synthesis of more effective spectinomycin compounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Antibiotics
医学-免疫学
CiteScore
6.60
自引率
3.00%
发文量
87
审稿时长
1 months
期刊介绍:
The Journal of Antibiotics seeks to promote research on antibiotics and related types of biologically active substances and publishes Articles, Review Articles, Brief Communication, Correspondence and other specially commissioned reports. The Journal of Antibiotics accepts papers on biochemical, chemical, microbiological and pharmacological studies. However, studies regarding human therapy do not fall under the journal’s scope. Contributions regarding recently discovered antibiotics and biologically active microbial products are particularly encouraged. Topics of particular interest within the journal''s scope include, but are not limited to, those listed below:
Discovery of new antibiotics and related types of biologically active substances
Production, isolation, characterization, structural elucidation, chemical synthesis and derivatization, biological activities, mechanisms of action, and structure-activity relationships of antibiotics and related types of biologically active substances
Biosynthesis, bioconversion, taxonomy and genetic studies on producing microorganisms, as well as improvement of production of antibiotics and related types of biologically active substances
Novel physical, chemical, biochemical, microbiological or pharmacological methods for detection, assay, determination, structural elucidation and evaluation of antibiotics and related types of biologically active substances
Newly found properties, mechanisms of action and resistance-development of antibiotics and related types of biologically active substances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


