驼科动物针对 C4-二羧酸盐转运体的单域抗体片段(VHH)可逆转铜绿假单胞菌对碳青霉烯类药物的耐药性。
IF 2.1
4区 医学
Q3 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
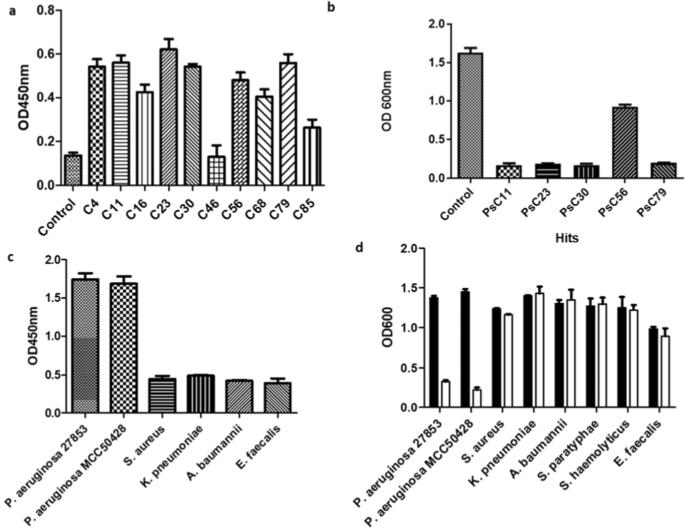
抗菌药耐药性正在成为新的医疗危机,因此有必要采用非传统方法开发新的药物类别。铜绿假单胞菌是引起医院内感染的最常见病原体之一,即使使用最后的一线药物碳青霉烯类也极难治疗。由于这种病原体能够对正在使用的新的小分子抗生素产生耐药性,因此人们尝试了一种新的生物方法,将抗体片段与碳青霉烯类和 β-内酰胺类药物结合起来作为辅助疗法,以持久解决这一问题。在这项研究中,我们开发了一个针对铜绿假单胞菌的驼科抗体片段(VHH)文库,并分离出了一个强效靶点--PsC23。质谱分析鉴定出该靶标是 C4-二羧酸盐转运体的一个组成部分,该转运体特别是在氧化应激条件下将代谢物供给乙醛酸分流。PsC23 在浓度为 1.66 µM(25 µg ml-1)时具有抑菌作用,与这两类药物联合用药时,在 100-200 nM(1.5-3.0 µg ml-1)的有效浓度下具有协同作用。在与美罗培南联用时,VHH 能完全清除对碳青霉烯耐药的铜绿假单胞菌全身感染的中性粒细胞小鼠的感染。PsC23 阻断乙醛酸分流会破坏能量转移,导致呼吸转向缺氧的 TCA 循环,从而抑制碳青霉烯类和β-内酰胺类药物的外流并增加自由基的生成,从而产生强烈的杀菌作用,逆转对多种不相关药物的耐药性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Reversal of carbapenem resistance in Pseudomonas aeruginosa by camelid single domain antibody fragment (VHH) against the C4-dicarboxylate transporter
Antimicrobial resistance is emerging as the new healthcare crisis necessitating the development of newer classes of drugs using non-traditional approaches. Pseudomonas aeruginosa, one of the most common pathogens involved in nosocomial infections, is extremely difficult to treat even with the last resort frontline drug, the carbapenems. As the pathogen has the ability to acquire resistance to new small-molecule antibiotics, being deployed, a novel biological approach has been tried using antibody fragments in combination with carbapenems and β-lactams as adjunct therapy for an enduring solution to the problem. In this study, we developed a camelid antibody fragment (VHH) library against P. aeruginosa and isolated a highly potent hit, PsC23. Mass spectrometry identified the target to be a component of the C4-dicarboxylate transporter that feeds metabolites to the glyoxylate shunt particularly under conditions of oxidative stress. PsC23 is bacteriostatic at a concentration of 1.66 µM (25 µg ml−1) and shows a synergistic effect with both the classes of drugs at an effective concentration of 100–200 nM (1.5–3.0 µg ml−1) when co administered with them. In combination with meropenem the VHH completely cleared the infection from a neutropenic mouse with a carbapenem-resistant P. aeruginosa systemic infection. Blocking the glyoxylate shunt by PsC23 resulted in disruption of energy transduction due to a respiratory shift to the oxygen-depleted TCA cycle causing inhibition of efflux and increased free radical generation from carbapenems and β-lactams exerting a strong bactericidal effect that reversed the resistance to multiple unrelated drugs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Antibiotics
医学-免疫学
CiteScore
6.60
自引率
3.00%
发文量
87
审稿时长
1 months
期刊介绍:
The Journal of Antibiotics seeks to promote research on antibiotics and related types of biologically active substances and publishes Articles, Review Articles, Brief Communication, Correspondence and other specially commissioned reports. The Journal of Antibiotics accepts papers on biochemical, chemical, microbiological and pharmacological studies. However, studies regarding human therapy do not fall under the journal’s scope. Contributions regarding recently discovered antibiotics and biologically active microbial products are particularly encouraged. Topics of particular interest within the journal''s scope include, but are not limited to, those listed below:
Discovery of new antibiotics and related types of biologically active substances
Production, isolation, characterization, structural elucidation, chemical synthesis and derivatization, biological activities, mechanisms of action, and structure-activity relationships of antibiotics and related types of biologically active substances
Biosynthesis, bioconversion, taxonomy and genetic studies on producing microorganisms, as well as improvement of production of antibiotics and related types of biologically active substances
Novel physical, chemical, biochemical, microbiological or pharmacological methods for detection, assay, determination, structural elucidation and evaluation of antibiotics and related types of biologically active substances
Newly found properties, mechanisms of action and resistance-development of antibiotics and related types of biologically active substances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


