基于电洗脱的共价 DNA 蛋白交联纯化。
IF 13.1
1区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
共价 DNA 蛋白交联(DPCs)是一种普遍存在的 DNA 病变,对基因组的稳定性构成挑战,可由代谢或化疗交联剂诱发,包括活性醛类、拓扑异构酶毒物和 DNMT1 抑制剂。通过电洗脱将 DNA 交联蛋白从可溶性蛋白中分离出来的 x 链接蛋白(PxP)纯化法可用于鉴定 DPC。在此,我们介绍一种多功能、灵敏的 PxP 方法。在暴露于 DPC 诱导剂后收集哺乳动物细胞,将其嵌入低熔点琼脂糖塞中,并在变性条件下进行裂解。裂解后,用电洗脱法从琼脂糖塞中提取可溶性蛋白质,而基因组 DNA 和交联蛋白质则保留在琼脂糖塞中。交联蛋白质可通过标准分析技术进行分析,如十二烷基硫酸钠-聚丙烯酰胺凝胶电泳,然后进行 Western 印迹或荧光染色。另外,还可以使用基于质谱的定量蛋白质组学方法对 DPC 进行无偏见的鉴定。通过 PxP 分离和分析 DPCs 克服了其他分析 DPCs 方法的局限性,这些方法依赖沉淀作为分离原理,受过分子或细胞生物学培训的用户可在 2-3 天内完成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
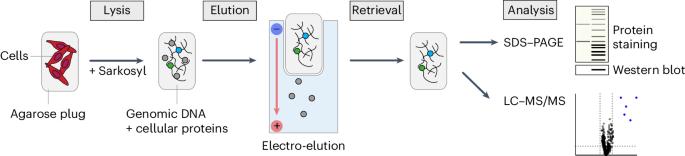
Electro-elution-based purification of covalent DNA–protein cross-links
Covalent DNA–protein cross-links (DPCs) are pervasive DNA lesions that challenge genome stability and can be induced by metabolic or chemotherapeutic cross-linking agents including reactive aldehydes, topoisomerase poisons and DNMT1 inhibitors. The purification of x-linked proteins (PxP), where DNA–cross-linked proteins are separated from soluble proteins via electro-elution, can be used to identify DPCs. Here we describe a versatile and sensitive strategy for PxP. Mammalian cells are collected following exposure to a DPC-inducing agent, embedded in low-melt agarose plugs and lysed under denaturing conditions. Following lysis, the soluble proteins are extracted from the agarose plug by electro-elution, while genomic DNA and cross-linked proteins are retained in the plug. The cross-linked proteins can then be analyzed by standard analytical techniques such as sodium dodecyl-sulfate–polyacrylamide gel electrophoresis followed by western blotting or fluorescent staining. Alternatively, quantitative mass spectrometry-based proteomics can be used for the unbiased identification of DPCs. The isolation and analysis of DPCs by PxP overcomes the limitations of alternative methods to analyze DPCs that rely on precipitation as the separating principle and can be performed by users trained in molecular or cell biology within 2–3 d. The protocol has been optimized to study DPC induction and repair in mammalian cells but may also be adapted to other sample types including bacteria, yeast and tissue samples. An assay based on the electrophoresis of whole-cell lysates embedded in agarose plugs separates soluble from immobilized proteins, enabling the purification and the subsequent identification of DNA–protein cross-links.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Nature Protocols
生物-生化研究方法
CiteScore
29.10
自引率
0.70%
发文量
128
审稿时长
4 months
期刊介绍:
Nature Protocols focuses on publishing protocols used to address significant biological and biomedical science research questions, including methods grounded in physics and chemistry with practical applications to biological problems. The journal caters to a primary audience of research scientists and, as such, exclusively publishes protocols with research applications. Protocols primarily aimed at influencing patient management and treatment decisions are not featured.
The specific techniques covered encompass a wide range, including but not limited to: Biochemistry, Cell biology, Cell culture, Chemical modification, Computational biology, Developmental biology, Epigenomics, Genetic analysis, Genetic modification, Genomics, Imaging, Immunology, Isolation, purification, and separation, Lipidomics, Metabolomics, Microbiology, Model organisms, Nanotechnology, Neuroscience, Nucleic-acid-based molecular biology, Pharmacology, Plant biology, Protein analysis, Proteomics, Spectroscopy, Structural biology, Synthetic chemistry, Tissue culture, Toxicology, and Virology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


