反选择催化
IF 38.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
异构体化合物--因围绕单键的旋转受限而产生的立体异构体--近年来引起了广泛关注,因为它们在药物化学、催化和分子纳米科学等多个领域具有巨大的应用潜力。这种日益增长的兴趣促使人们发明了新的分子马达,将阿托异构体纳入了药物发现计划,并产生了一系列新型的阿托选择性反应,包括同时控制多个立体轴的反应。在催化剂控制下立体选择性合成各类对位异构体的方法多种多样,可分为非对称反应、(动态)动力学解析、交叉偶联反应和全新成环反应。在本综述中,我们概括了催化剂控制立体选择性合成这些类别中的异构体的概念,重点是立体生成的 C(sp2)-C(sp2) 轴以外的最新进展和未充分开发的异构体支架。我们还讨论了具有多个立体构象轴或高阶立体构象的更复杂系统。本文章由计算机程序翻译,如有差异,请以英文原文为准。
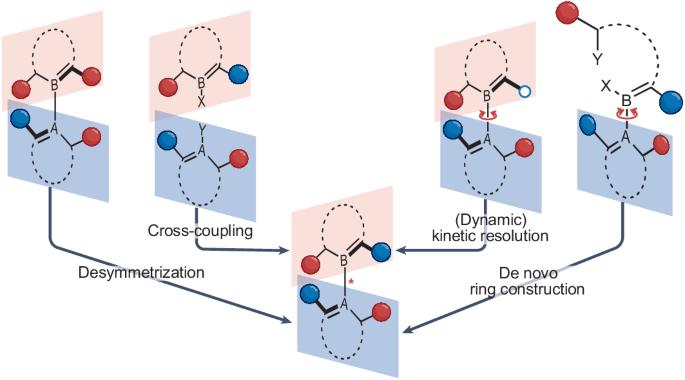
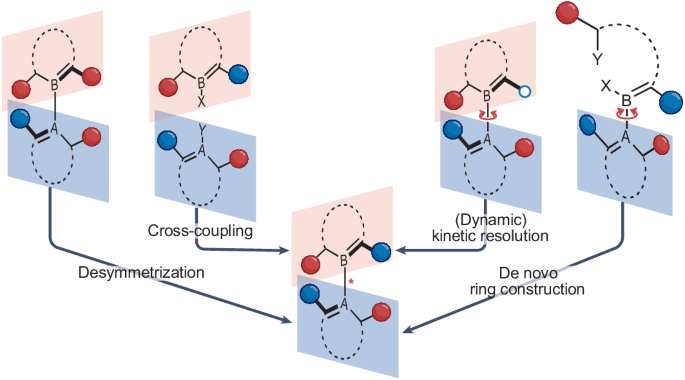
Atroposelective catalysis
Atropisomeric compounds—stereoisomers that arise from the restricted rotation about a single bond—have attracted widespread attention in recent years due to their immense potential for applications in a variety of fields, including medicinal chemistry, catalysis and molecular nanoscience. This increased interest led to the invention of new molecular motors, the incorporation of atropisomers into drug discovery programmes and a wide range of novel atroposelective reactions, including those that simultaneously control multiple stereogenic axes. A diverse set of synthetic methodologies, which can be grouped into desymmetrizations, (dynamic) kinetic resolutions, cross-coupling reactions and de novo ring formations, is available for the catalyst-controlled stereoselective synthesis of various atropisomer classes. In this Review, we generalize the concepts for the catalyst-controlled stereoselective synthesis of atropisomers within these categories with an emphasis on recent advancements and underdeveloped atropisomeric scaffolds beyond stereogenic C(sp2)–C(sp2) axes. We also discuss more complex systems with multiple stereogenic axes or higher-order stereogenicity. The catalyst-controlled stereoselective synthesis of atropisomers is feasible by four main concepts: desymmetrization, (dynamic) kinetic resolution, direct formation of the stereogenic axis and de novo ring construction. In this Review, pioneering work in atroposelective catalysis is discussed alongside recent advances.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature reviews. Chemistry
Chemical Engineering-General Chemical Engineering
CiteScore
52.80
自引率
0.80%
发文量
88
期刊介绍:
Nature Reviews Chemistry is an online-only journal that publishes Reviews, Perspectives, and Comments on various disciplines within chemistry. The Reviews aim to offer balanced and objective analyses of selected topics, providing clear descriptions of relevant scientific literature. The content is designed to be accessible to recent graduates in any chemistry-related discipline while also offering insights for principal investigators and industry-based research scientists. Additionally, Reviews should provide the authors' perspectives on future directions and opinions regarding the major challenges faced by researchers in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


