在成人中使用肠外鱼油脂质乳剂:一个肠道功能衰竭转诊中心的病例系列和回顾。
IF 3.6
3区 医学
Q2 NUTRITION & DIETETICS
引用次数: 0
摘要
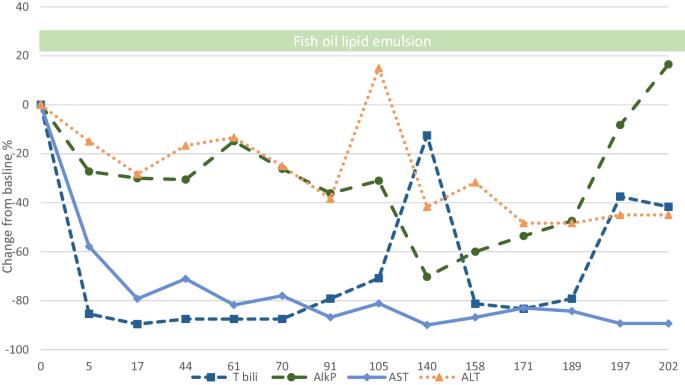
背景:肠功能衰竭相关性肝病(IFALD)是长期使用 PN 的一种并发症,归因于ω-6 注射用脂质乳剂(ILE)的使用。鱼油 ILE 成功逆转了新生儿的肝损伤。在成年患者中使用纯鱼油 ILE 的证据有限:方法:芝加哥大学新生儿营养登记处对患有 IFALD 的成人患者使用 FO 脂质乳剂的病例系列。对病历和 PN 配方进行了分析:结果:发现了三例使用FO ILE治疗的IFALD病例。第一个病例是一名 30 岁的男性,患有短肠综合征 (SBS)、高胆红素血症和活组织检查证实的 IFALD。在从大豆脂质乳剂换成膳食纤维脂质乳剂后,他的肝脏检查迅速得到改善,并在使用 202 周后保持稳定。第二个病例是一名 76 岁的妇女,因肠冻死而导致肠功能衰竭(IF)。从大豆 ILE 到复合脂质,再到后来的纯 FO ILE,她的肝脏测试结果都没有改善。第三个病例是一名 28 岁的男性,患有 SBS 和活组织检查证实的 IFALD。改用复合 ILE 后,又改用 FO 脂质乳剂,结果肝脏测试逐渐得到改善。治疗期间未发现临床必需脂肪酸(EFA)缺乏症:结论:FO ILE 可有效治疗患有胆汁淤积性 IFALD 的成年患者。结论:FO ILE 可有效治疗患有胆汁淤积性 IFALD 的成年患者,而且使用安全,在长达 4 年的使用过程中未发现 EFA 缺乏症。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Parenteral fish oil lipid emulsion use in adults: a case series and review from an intestinal failure referral center
Intestinal failure-associated liver disease (IFALD) is a complication of long-term PN use, attributed to the use of ω-6 injectable lipid emulsions (ILE). Fish oil (FO) ILE have been successful in reversing liver injury in neonates. Evidence for pure FO ILE use in adult patients is limited. Case series of the use of FO lipid emulsions in adults with IFALD from the University of Chicago PN registry. Analysis of medical charts and PN formulations was performed. Three cases of IFALD treated with FO ILE were identified. The first case was a 30-year-old man with short bowel syndrome (SBS), hyperbilirubinemia, and biopsy-proven IFALD. Following a change from a soy lipid emulsion to FO lipid emulsion, his liver tests rapidly improved and remained stable over 202 weeks of use. The second case was a 76-year-old woman with intestinal failure (IF) due to a frozen bowel. A change from a soy ILE to a composite lipid and later to a pure FO ILE did not result in improvement in her liver tests. The third case was a 28-year-old man with SBS and biopsy-proven IFALD. Change to a composite ILE and subsequently FO lipid emulsion resulted in a gradual improvement in liver tests. No clinical essential fatty acid (EFA) deficiencies were identified during treatment. FO ILE may be effective in the treatment of adult patients with cholestatic IFALD. Use is safe with no EFA deficiencies detected in up to 4 years of use.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.60
自引率
2.10%
发文量
189
审稿时长
3-6 weeks
期刊介绍:
The European Journal of Clinical Nutrition (EJCN) is an international, peer-reviewed journal covering all aspects of human and clinical nutrition. The journal welcomes original research, reviews, case reports and brief communications based on clinical, metabolic and epidemiological studies that describe methodologies, mechanisms, associations and benefits of nutritional interventions for clinical disease and health promotion.
Topics of interest include but are not limited to:
Nutrition and Health (including climate and ecological aspects)
Metabolism & Metabolomics
Genomics and personalized strategies in nutrition
Nutrition during the early life cycle
Health issues and nutrition in the elderly
Phenotyping in clinical nutrition
Nutrition in acute and chronic diseases
The double burden of ''malnutrition'': Under-nutrition and Obesity
Prevention of Non Communicable Diseases (NCD)

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


