以线粒体为靶点的Ir(III)复合物可引发铁变态反应和自噬,用于癌症治疗:金属复合物的聚集增强型 PDT 策略案例
IF 9.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
金属复合物在肿瘤诊断和治疗中大有可为。然而,它们在光动力疗法(PDT)中的潜在应用却受到光稳定性差、活性氧(ROS)产量低以及聚集诱导的 ROS淬灭等问题的阻碍。为了应对这些挑战,我们提出了一种利用聚集诱导发射(AIE)共轭金属复合物的分子自组装策略。作为概念验证,我们合成了一种线粒体靶向环甲基化 Ir(III) 光敏剂 Ir-TPE。这种方法大大增强了光动力效应,同时减轻了与 AIE 基团相关的暗毒性。Ir-TPE 在水溶液中很容易自组装成纳米团聚体,从而在光照射时产生大量 ROS。经过光照射的 Ir-TPE 会在线粒体中过度积累 ROS,导致线粒体 DNA 损伤,从而引发多种死亡模式。这种损伤可导致铁突变和自噬,这两种细胞死亡方式对癌细胞具有很强的细胞毒性。Ir-TPE 的聚集增强型光动力效应可显著提高 ROS 的产生,从而产生更明显的细胞毒性效应。体外和体内实验证明,这种聚集增强型光动力疗法能有效地在原位根除肿瘤。这项研究不仅解决了金属复合物因聚集而产生较少 ROS 的局限性,还凸显了这一策略在增强 PDT 中产生 ROS 的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
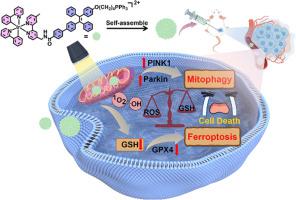
A mitochondria targeting Ir(III) complex triggers ferroptosis and autophagy for cancer therapy: A case of aggregation enhanced PDT strategy for metal complexes
Metal complexes hold significant promise in tumor diagnosis and treatment. However, their potential applications in photodynamic therapy (PDT) are hindered by issues such as poor photostability, low yield of reactive oxygen species (ROS), and aggregation-induced ROS quenching. To address these challenges, we present a molecular self-assembly strategy utilizing aggregation-induced emission (AIE) conjugates for metal complexes. As a proof of concept, we synthesized a mitochondrial-targeting cyclometalated Ir(III) photosensitizer Ir-TPE. This approach significantly enhances the photodynamic effect while mitigating the dark toxicity associated with AIE groups. Ir-TPE readily self-assembles into nanoaggregates in aqueous solution, leading to a significant production of ROS upon light irradiation. Photoirradiated Ir-TPE triggers multiple modes of death by excessively accumulating ROS in the mitochondria, resulting in mitochondrial DNA damage. This damage can lead to ferroptosis and autophagy, two forms of cell death that are highly cytotoxic to cancer cells. The aggregation-enhanced photodynamic effect of Ir-TPE significantly enhances the production of ROS, leading to a more pronounced cytotoxic effect. In vitro and in vivo experiments demonstrate this aggregation-enhanced PDT approach achieves effective in situ tumor eradication. This study not only addresses the limitations of metal complexes in terms of low ROS production due to aggregation but also highlights the potential of this strategy for enhancing ROS production in PDT.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


