钯催化咔唑与炔基溴化物的直接炔基化反应
IF 2.5
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
咔唑衍生物在药物化学和材料化学中至关重要,但获得多样化的取代仍然具有挑战性。在这里,我们介绍了一种通过钯催化 C-H 活化咔唑的 C1 和 C8 烷炔化的区域选择性方法。该方法提供了获得单炔基化和二炔基化衍生物的直接途径,解决了炔基化咔唑基材料稀缺的问题。我们的研究拓宽了咔唑官能化的合成工具箱,为光电子学、生物成像等领域提供了潜在应用,同时有助于开发可持续的 C-H 活化方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
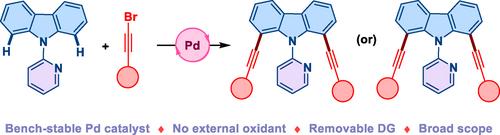
Palladium‐Catalyzed Direct Alkynylation of Carbazoles with Alkynyl Bromides
Carbazole derivatives are vital in medicinal and materials chemistry, yet accessing diverse substitutions remains challenging. Here, we introduce a regioselective approach for C1 and C8 alkynylation of carbazoles via palladium‐catalyzed C−H activation. This method provides a direct route to mono‐ and di‐alkynylated derivatives, addressing the scarcity of alkynylated carbazole‐based materials. Our study broadens the synthetic toolbox for carbazole functionalization, offering potential applications in optoelectronics, bio‐imaging, and beyond, while contributing to developing sustainable C−H activation methodologies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.40
自引率
3.60%
发文量
752
审稿时长
1 months
期刊介绍:
The European Journal of Organic Chemistry (2019 ISI Impact Factor 2.889) publishes Full Papers, Communications, and Minireviews from the entire spectrum of synthetic organic, bioorganic and physical-organic chemistry. It is published on behalf of Chemistry Europe, an association of 16 European chemical societies.
The following journals have been merged to form two leading journals, the European Journal of Organic Chemistry and the European Journal of Inorganic Chemistry:
Liebigs Annalen
Bulletin des Sociétés Chimiques Belges
Bulletin de la Société Chimique de France
Gazzetta Chimica Italiana
Recueil des Travaux Chimiques des Pays-Bas
Anales de Química
Chimika Chronika
Revista Portuguesa de Química
ACH—Models in Chemistry
Polish Journal of Chemistry.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


