碱性/天然海水的电还原:自清洁铂/碳阴极和 H2 与氢氧化镁纳米片的现场共合成
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
分布式沿海/近海海水分离厂可促进基于 H2 的经济在全球范围内的应用。越来越多的研究主要集中于抑制卤化离子的阳极氧化。同样,由于大多数研究都使用碱性海水,自然海水还原(NSR)中棘手的阴极沉淀问题也被忽视了。在此,我们探讨了减轻表面沉淀的可能策略(引入质子海绵以改变阴极微环境、打破局部 OH- 梯度、采用自清洁阴极)。我们将一种著名的具有 H2 演化活性的金属--铂--引入到具有 H2 气体排空能力的自清洁碳支持物中。我们提出的无粘结剂铂/碳阴极比以前许多用于 NSR 的铂/碳阴极更坚固。此外,我们还强调了从天然海水中共同电合成纳米级氢氧化镁和 H2 的可能性。这项工作表明,局部环境设计、pH 梯度破坏和/或基于阴极结构的气体/液体流动可以抑制表面沉淀。我们详细展示了 NSR 中的各种问题和可能的解决方案。本文章由计算机程序翻译,如有差异,请以英文原文为准。
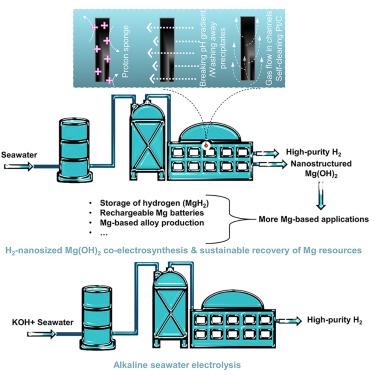
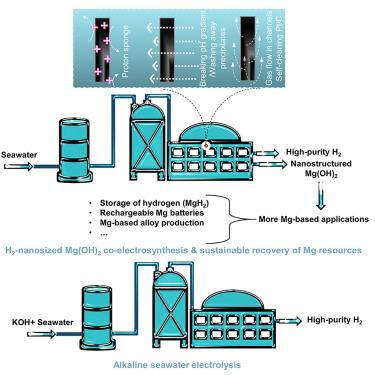
Electroreduction of alkaline/natural seawater: Self-cleaning Pt/carbon cathode and on-site co-synthesis of H2 and Mg hydroxide nanoflakes
Distributed coastal/offshore seawater splitting plants can facilitate H2-based economy’s global deployment. Increasingly, studies emerge mostly focusing on inhibiting anodic oxidation of halide ions. Equally tricky cathodic precipitation in natural seawater reduction (NSR) is neglected due to the use of alkaline seawater in most studies. Herein, we explore possible strategies (introducing a proton sponge to change cathodic microenvironments, breaking local OH− gradients, employing self-cleaning cathodes) to alleviate surface precipitation. We introduce a famous H2 evolution-active metal, Pt, onto a self-cleaning carbon support with H2 gas evacuation capability. Our proposed binder-free Pt/carbon cathode is more robust than many previous Pt/C cathodes for NSR. Moreover, we highlight possibilities of co-electrosynthesizing nano-sized Mg hydroxides and H2 from natural seawater. This work suggests that designs of local environments, pH gradient disruption, and/or cathode architecture-based gas/liquid flows may suppress surface precipitation. We demonstrate in detail the various issues in NSR and possible solutions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


