内体中介导蛋白质分拣的管状载体的组装和裂变
IF 81.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
在所有真核生物中,内体都是蛋白质分拣的中心站,处于众多膜运输途径的十字路口。它们在蛋白质平衡和细胞信号传导中起着关键作用,并与多种疾病的发病机制有关。内含体相关蛋白集合体或包膜收集跨膜货物蛋白,并将它们集中到检索域中。这些结构域可延伸为管状载体,然后从内体膜上挤压下来,将货物运送到适当的亚细胞区。在这里,我们讨论了对一些管状膜包被结构的新见解,这些包被介导了将货物招募到这些载体中,重点是基于分拣神经蛋白的包被,如 Retromer、Commander 和 ESCPE-1。我们总结了关于选择性管状内体载体如何通过裂变形成和脱离内体的现有观点和新观点,重点介绍了结构方面、概念上的挑战和开放性问题。本文章由计算机程序翻译,如有差异,请以英文原文为准。
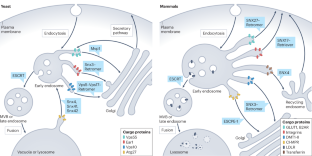
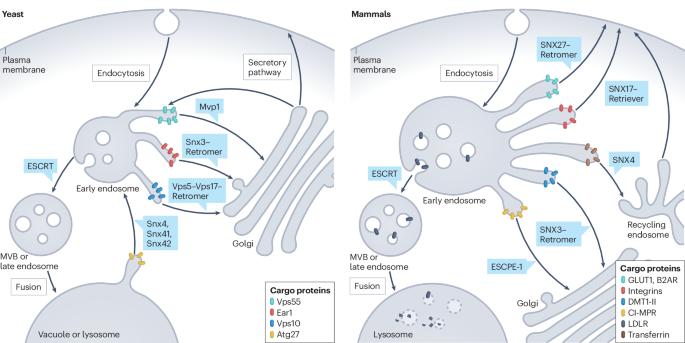
Assembly and fission of tubular carriers mediating protein sorting in endosomes
Endosomes are central protein-sorting stations at the crossroads of numerous membrane trafficking pathways in all eukaryotes. They have a key role in protein homeostasis and cellular signalling and are involved in the pathogenesis of numerous diseases. Endosome-associated protein assemblies or coats collect transmembrane cargo proteins and concentrate them into retrieval domains. These domains can extend into tubular carriers, which then pinch off from the endosomal membrane and deliver the cargoes to appropriate subcellular compartments. Here we discuss novel insights into the structure of a number of tubular membrane coats that mediate the recruitment of cargoes into these carriers, focusing on sorting nexin-based coats such as Retromer, Commander and ESCPE-1. We summarize current and emerging views of how selective tubular endosomal carriers form and detach from endosomes by fission, highlighting structural aspects, conceptual challenges and open questions. Endosomes function as sorting stations that segregate cargo proteins into endosomal carriers, enabling their distribution to subcellular target compartments. Increasingly detailed structural insights have revealed how proteins, such as sorting nexins, assemble on endosomal membranes to form a coat that facilitates the formation and detachment of tubular carriers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
173.60
自引率
0.50%
发文量
118
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Molecular Cell Biology is a prestigious journal that aims to be the primary source of reviews and commentaries for the scientific communities it serves. The journal strives to publish articles that are authoritative, accessible, and enriched with easily understandable figures, tables, and other display items. The goal is to provide an unparalleled service to authors, referees, and readers, and the journal works diligently to maximize the usefulness and impact of each article. Nature Reviews Molecular Cell Biology publishes a variety of article types, including Reviews, Perspectives, Comments, and Research Highlights, all of which are relevant to molecular and cell biologists. The journal's broad scope ensures that the articles it publishes reach the widest possible audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


