天然不育麝香杂交种适合用于产生假孕胚胎移植受体
IF 5.9
3区 农林科学
Q1 VETERINARY SCIENCES
引用次数: 0
摘要
在胚胎移植受体的准备过程中,通常使用输精管结扎小鼠,手术过程会带来疼痛和不适。无菌转基因品系提供了一种非手术替代方法,但其维持需要育种和基因分型程序。我们以前曾报道过使用自然不育的 STUSB6F1 杂交小鼠生产胚胎移植受体,发现这些受体的行为与输精管结扎雄性小鼠产生的受体没有区别。该方法提供了两个实质性的 3R 影响:精细化(与外科输精管结扎术相比)和减少育种程序(与不育转基因品系相比)。尽管最初前景看好,但由于亲本 STUS/Fore 品系的培育困难重重,不育杂交种无法在更大范围内推广,这项创新的 3R 影响受到了限制。3R 计划的价值取决于社区的接受程度。因此,我们在此选择了一种不同的自然不育杂交种,该杂交种由可广泛获得的菌株产生:C57BL/6J与Mus spretus的B6SPRTF1杂交种。我们首先通过精子计数和睾丸重量确认了它的不育性,然后在英国的三个设施中试用了低温保存胚胎和种质的回收。通过体外受精产生这些杂交种的精子分配方法被认为是最可靠的分配方法,而且无需维持一个活的M. spretus群体。然后,我们测试了 B6SPRTF1 不育杂交种在这三个英国设施中用于胚胎移植受体的适宜性,结果发现,与手术输精管结扎小鼠和不育转基因品系相比,这些杂交种是适宜的。总之,无菌 B6SPRTF1 杂交种在独立生产设施中的易分配性和实用性证实了这种方法潜在的 3Rs 影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
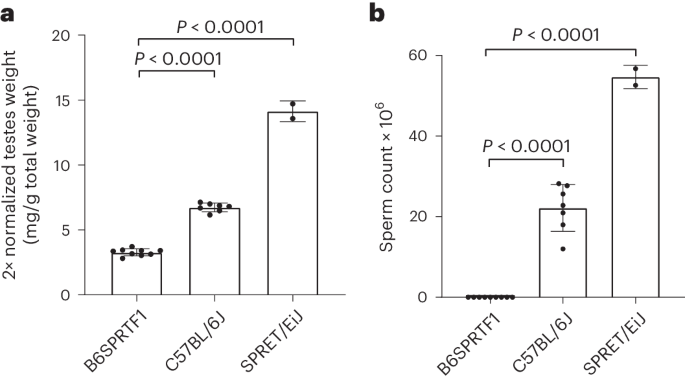
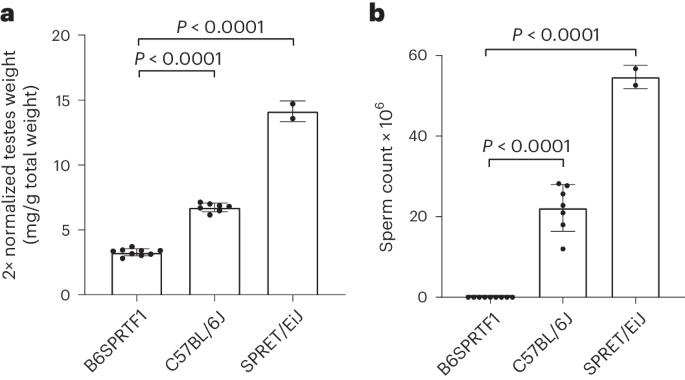
Naturally sterile Mus spretus hybrids are suitable for the generation of pseudopregnant embryo transfer recipients
For the preparation of embryo transfer recipients, surgically vasectomized mice are commonly used, generated by procedures associated with pain and discomfort. Sterile transgenic strains provide a nonsurgical replacement, but their maintenance requires breeding and genotyping procedures. We have previously reported the use of naturally sterile STUSB6F1 hybrids for the production of embryo transfer recipients and found the behavior of these recipients to be indistinguishable from those generated by vasectomized males. The method provides two substantial 3R impacts: refinement (when compared with surgical vasectomy) and reduction in breeding procedures (compared with sterile transgenic lines). Despite initial promise, the 3Rs impact of this innovation was limited by difficulties in breeding the parental STUS/Fore strain, which precluded the wider distribution of the sterile hybrid. The value of a 3R initiative is only as good as the uptake in the community. Here we, thus, select a different naturally sterile hybrid, generated from strains that are widely available: the B6SPRTF1 hybrid between C57BL/6J and Mus spretus. We first confirmed its sterility by sperm counting and testes weight and then trialed the recovery of cryopreserved embryos and germplasm within three UK facilities. Distribution of sperm for the generation of these hybrids by in vitro fertilization was found to be the most robust distribution method and avoided the need to maintain a live M. spretus colony. We then tested the suitability of B6SPRTF1 sterile hybrids for the generation of embryo transfer recipients at these same three UK facilities and found the hybrids to be suitable when compared with surgical vasectomized mice and a sterile transgenic strain. In conclusion, the potential 3Rs impact of this method was confirmed by the ease of distribution and the utility of sterile B6SPRTF1 hybrids at independent production facilities. Preece et al. show that B6SPRTF1 hybrid males between C57BL/6J and Mus spretus are suitable for the generation of pseudopregnant female mice for embryo transfer. By providing an alternative to vasectomized males, the method shows clear 3R benefits.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Lab Animal
农林科学-兽医学
CiteScore
0.60
自引率
2.90%
发文量
181
审稿时长
>36 weeks
期刊介绍:
LabAnimal is a Nature Research journal dedicated to in vivo science and technology that improves our basic understanding and use of model organisms of human health and disease. In addition to basic research, methods and technologies, LabAnimal also covers important news, business and regulatory matters that impact the development and application of model organisms for preclinical research.
LabAnimal's focus is on innovative in vivo methods, research and technology covering a wide range of model organisms. Our broad scope ensures that the work we publish reaches the widest possible audience. LabAnimal provides a rigorous and fair peer review of manuscripts, high standards for copyediting and production, and efficient publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


