通过 N-杂环羰基催化不对称合成 C3 功能化 2,1-苯并噻嗪 2,2-二氧化物
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
我们在此介绍一种利用 N-杂环碳烯(NHC)催化对 2,1-苯并噻嗪 2,2- 二氧杂环进行对映选择性 C3 功能化的方法。我们的方法包括与不同的 N 型和 O 型亲核物进行一系列 [3+3] 环加成和开环反应,然后进行硅烷化。该方法克服了针对 C3 位的选择性难题,具有广泛的底物范围和高对映选择性。这标志着 NHC 催化转化领域的重大进展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
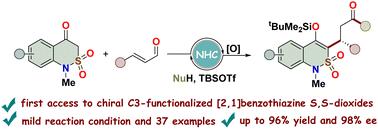
Enantioselective synthesis of C3-functionalized 2,1-benzothiazine 2,2-dioxides by N-heterocyclic carbene catalysis†
We present herein an approach for the enantioselective C3-functionalization of 2,1-benzothiazine 2,2-dioxides using N-heterocyclic carbene (NHC) catalysis. Our method involves a sequence of [3+3] cycloaddition and ring-opening reactions with different N- and O-nucleophiles, followed by silylation. Overcoming the challenge of selectivity targeting the C3 position, this protocol demonstrates a broad substrate scope and high enantioselectivity. This marks a significant advancement in the field of NHC-catalyzed transformations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


