通过 Cas9 介导的天然变体插入造血干细胞和祖细胞,提高红细胞生成量
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
一些基因多态性可导致单基因疾病,而另一些基因多态性则可能赋予人有益的性状。先天性红细胞增多症(非致病性红细胞生成过多症)就是一个典型的例子,它是由截短的促红细胞生成素受体引起的。在这里,我们展示了在 CD34+ 人类造血干细胞和祖细胞(HSPCs)中进行 Cas9 介导的基因组编辑可以重新创建红细胞生成素受体的截短形式,从而大幅增加红细胞生成量。我们还发现,将表达截短型促红细胞生成素受体的 cDNA 与之前报道的基因组编辑策略相结合,在 HSPCs 中用 HBB 转基因完全取代 HBA1 基因(以恢复β-地中海贫血表型细胞的正常血红蛋白生成),可使编辑后的 HSPCs 和健康红细胞表型具有增殖优势。将人类遗传学知识与精确的基因组编辑技术相结合,在治疗细胞中插入天然人类变异体,可为遗传病患者提供更安全、更有效的基因组编辑疗法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
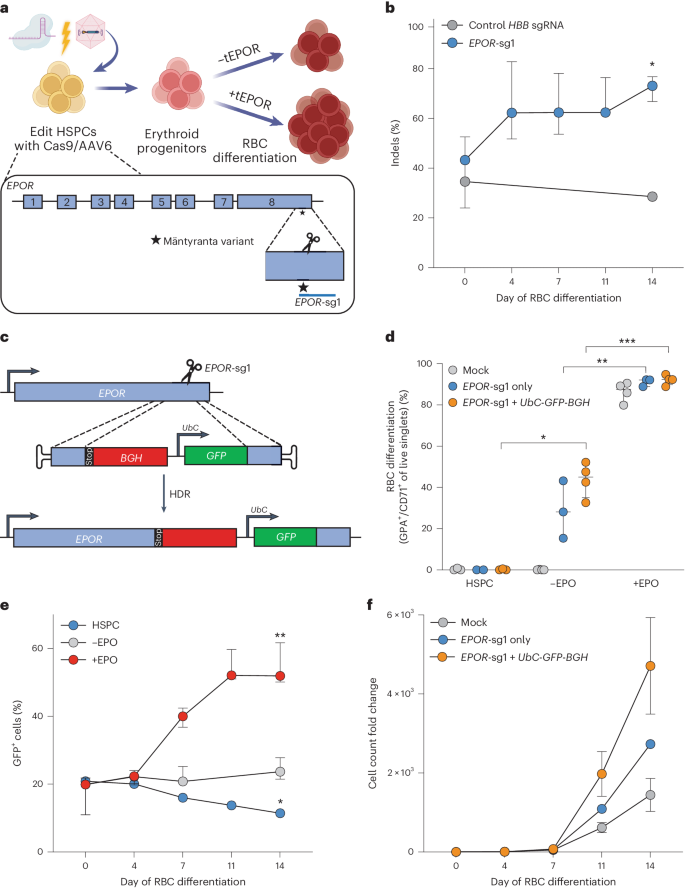

Enhancement of erythropoietic output by Cas9-mediated insertion of a natural variant in haematopoietic stem and progenitor cells
Some gene polymorphisms can lead to monogenic diseases, whereas other polymorphisms may confer beneficial traits. A well-characterized example is congenital erythrocytosis—the non-pathogenic hyper-production of red blood cells—that is caused by a truncated erythropoietin receptor. Here we show that Cas9-mediated genome editing in CD34+ human haematopoietic stem and progenitor cells (HSPCs) can recreate the truncated form of the erythropoietin receptor, leading to substantial increases in erythropoietic output. We also show that combining the expression of the cDNA of a truncated erythropoietin receptor with a previously reported genome-editing strategy to fully replace the HBA1 gene with an HBB transgene in HSPCs (to restore normal haemoglobin production in cells with a β-thalassaemia phenotype) gives the edited HSPCs and the healthy red blood cell phenotype a proliferative advantage. Combining knowledge of human genetics with precise genome editing to insert natural human variants into therapeutic cells may facilitate safer and more effective genome-editing therapies for patients with genetic diseases. Recreation of the truncated form of the erythropoietin receptor in human haematopoietic stem and progenitor cells via Cas9-mediated genome editing gives the edited cells and their erythropoietic progeny a proliferative advantage.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


