通过 Ugi 叠氮化物/多米诺过程耦合策略,超声辅助非对映选择性绿色合成以酰胺键杂环生物异构体功能化的螺融-γ-内酰胺。
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
通过基于异氰酸酯的多组分反应(IMCR)合成了一系列连接型 1,5-二取代四唑(1,5-DS-Ts),并以此为合成平台,在绿色条件下获得了含有环氧异吲哚-1(6H)-酮支架的结合型多杂环。这种快速声化学合成策略包括一个双多米诺过程,在乌基叠氮(UA)反应中使用一个正交杂环输入。通过 DFT 计算和 NBO 分析,我们了解了 UA 机理中 1,5 电环化所涉及的假环反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
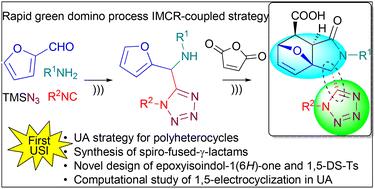
Ultrasound-assisted diastereoselective green synthesis of spiro-fused-γ-lactams functionalized with an amide bond heterocyclic bioisostere via the Ugi azide/domino process coupled strategy†
A series of linked-type 1,5-disubstituted tetrazoles (1,5-DS-Ts) were synthesised via an isocyanide-based multicomponent reaction (IMCR) and used as synthetic platforms to access bound-type polyheterocycles containing an epoxyisoindol-1(6H)-one scaffold under green conditions. This rapid sonochemical synthetic strategy includes a double domino process using an orthogonal heterocyclic input in the Ugi-azide (UA) reaction. DFT calculations and NBO analysis were performed to understand the pseudopericyclic reaction involved in the 1,5-electrocyclization of the UA mechanism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
The international home of synthetic, physical and biomolecular organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


