源于生物的介硅酸盐 SBA-15:合成、表征及在重金属去除方面的应用
IF 10.4
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
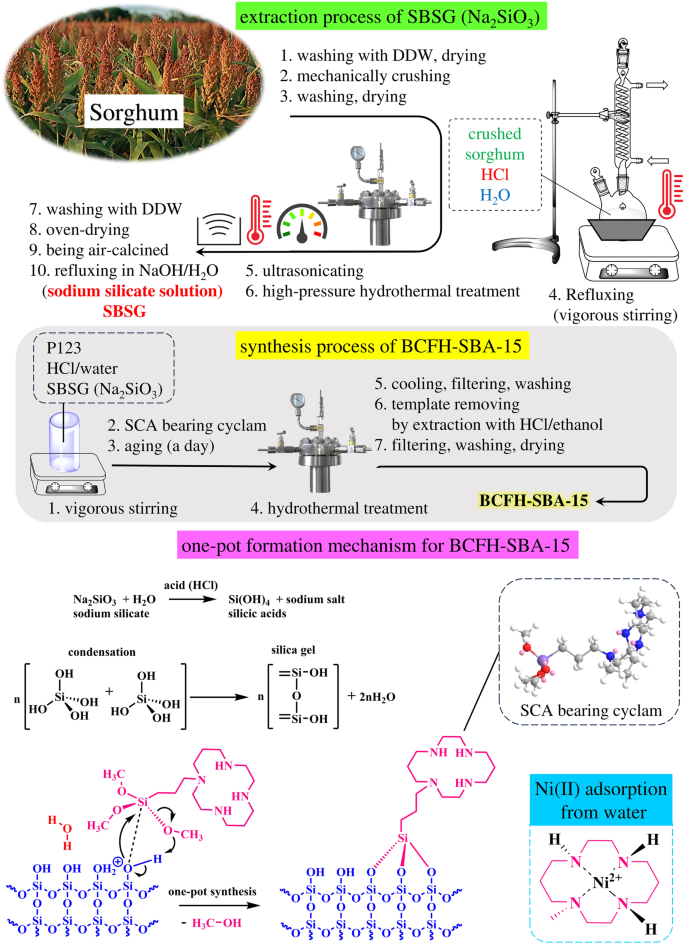
在以可持续和生态友好的生产方法制造纳米材料并将其用于环境应用的道路上,本文论述了一种生物源环己烷功能化同源 SBA-15 (BCFH-SBA-15)的成功合成。为此,本文采用简单的一锅式环保方法,将大量食用的高粱农业废弃物作为丰富的二氧化硅来源,制备出具有双峰微多孔结构和 325 平方米 g-1 大表面积的 BCFH-SBA-15。通过不同的仪器分析,如 XRD、FTIR、FESEM、TEM 和氮吸附/解吸等温线,对该材料进行了结构表征。事实证明,BCFH-SBA-15 能高效吸附水溶液中的镍(II),这一点已被用于确定等温线、热力学和动力学吸附参数的最可靠的经典模型所证实。Langmuir 等温线模型最精确地反映了实验结果,并用于计算 BCFH-SBA-15 在最佳条件下(pH = 6.0、吸附剂剂量 = 3.00 毫克、接触时间 = 20 分钟)的最大吸附容量。在 298、303、308 和 313 K 四种温度下的最大吸附容量分别为 243.36、253.87、260.95 和 266.28 mg g-1,超过了之前报道的大多数镍(II)吸附剂。镍(II)在 BCFH-SBA-15 上吸附的热力学数据表明,该吸附过程具有很强的化学吸附性($${\triangle H}_{\rm{ads}}.}^{circ }$$ = +122.61 kJ mol-1)和自发过程($${\triangle G}_{\rm{ads}}.}^{\circ }$ .= -29.161 至 -36.801 kJ mol-1),随机性较低($${\triangle S}_{{\rm{ads}}.}^{\circ }$ . = 0.5093 kJ mol-1 K-1)。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Bio-originated mesosilicate SBA-15: synthesis, characterization, and application for heavy metal removal
In the path of walking on the road of sustainable and eco-friendly production methods for manufacturing nanomaterials and utilizing them in environmental applications, this article deals with the prosperous synthesis of a biogenic cyclam-functionalized homologous SBA-15 (BCFH-SBA-15). For this purpose, the agricultural waste of the extensively consumed sorghum was used as a rich source of silica in the preparation of BCFH-SBA-15 with a bimodal micro-mesoporous architecture and a substantial surface area of 325 m2 g–1 through a simple one-pot environmentally friendly approach. The material was structurally characterized through the use of different instrumental analyses such as XRD, FTIR, FESEM, TEM, and nitrogen adsorption/desorption isotherms. BCFH-SBA-15 proved to be highly efficient in adsorbing Ni(II) in aqueous solutions, as confirmed by the most reliable classical models utilized for determining isotherm, thermodynamic, and kinetic adsorption parameters. The Langmuir isotherm model provided the most accurate representation of the experimental results, and it was used to calculate the maximum adsorption capacity of BCFH-SBA-15 under optimal conditions (pH = 6.0, adsorbent dose = 3.00 mg, contact time = 20 min). The maximum adsorption capacity at four temperatures of 298, 303, 308, and 313 K was estimated to be 243.36, 253.87, 260.95, and 266.28 mg g–1, respectively; surpassing most previously reported adsorbents for Ni(II) adsorption. The thermodynamic data of Ni(II) adsorption on the BCFH-SBA-15 indicated a strong chemisorption ( $${\triangle H}_{{\rm{ads}}.}^{\circ }$$ = +122.61 kJ mol–1) and spontaneous process ( $${\triangle G}_{{\rm{ads}}.}^{\circ }$$ .= −29.161 to −36.801 kJ mol–1) with a low degree of randomness ( $${\triangle S}_{{\rm{ads}}.}^{\circ }$$ . = 0.5093 kJ mol–1 K–1).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

npj Clean Water
Environmental Science-Water Science and Technology
CiteScore
15.30
自引率
2.60%
发文量
61
审稿时长
5 weeks
期刊介绍:
npj Clean Water publishes high-quality papers that report cutting-edge science, technology, applications, policies, and societal issues contributing to a more sustainable supply of clean water. The journal's publications may also support and accelerate the achievement of Sustainable Development Goal 6, which focuses on clean water and sanitation.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


