以 Zn2+ 为介导的催化技术实现水性 Zn 离子电池的快速充电
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
可充电锌离子水电池(AZIBs)以其安全性、高能量密度和快速充电而著称,是电网规模能源存储的首选。从历史上看,以离子迁移行为为核心的离子跃迁模型一直主导着对水电池充放电过程的解释,如经典的离子插入/抽出和假电容机制。然而,与其他水性金属离子电池相比,这些模型难以解释 AZIB 的优异性能。在此,我们提出一种催化模型,通过催化中的吸附概念来解释水电池中的 Zn2+ 异常现象。这种行为可以起到充电/放电的作用,主要由溶解的金属阳离子和阴极材料决定。第一原理计算表明,Zn2+-氮化钒(VN)组合具有最佳的吸附/解吸行为(水解离过程)。在实验中,采用氮化钒阴极的 AZIBs 显示出快速充电动力学,在电流密度为 300,000 mA g-1 时,容量为 577.1 mAh g-1。掌握 AZIB 内的催化步骤可以推动解决方案超越最先进的快速充电电池。本文章由计算机程序翻译,如有差异,请以英文原文为准。
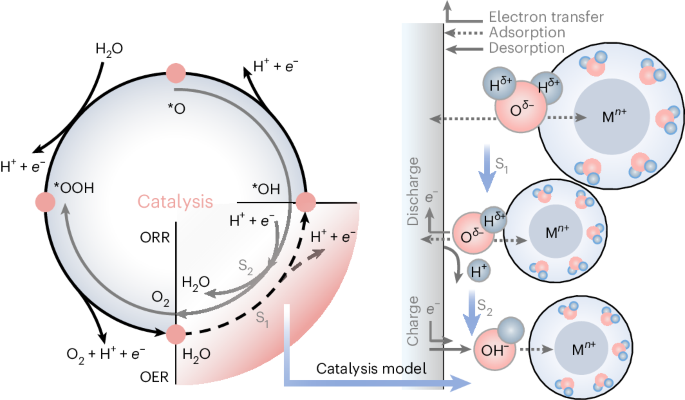
Zn2+-mediated catalysis for fast-charging aqueous Zn-ion batteries
Rechargeable aqueous zinc-ion batteries (AZIBs), renowned for their safety, high energy density and rapid charging, are prime choices for grid-scale energy storage. Historically, ion-shuttling models centring on ion-migration behaviour have dominated explanations for charge/discharge processes in aqueous batteries, like classical ion insertion/extraction and pseudocapacitance mechanisms. However, these models struggle to account for the exceptional performance of AZIBs compared to other aqueous metal-ion batteries. Here we present a catalysis model elucidating the Zn2+ anomaly in aqueous batteries, explaining it through the concept of adsorption in catalysis. Such behaviour can serve the charge/discharge role, predominantly dictated by solvated metal cations and cathode materials. First-principles calculations suggest optimal adsorption/desorption behaviour (water dissociation process) with the Zn2+–vanadium nitride (VN) combination. Experimentally, AZIBs implementing VN cathodes demonstrate fast-charging kinetics, showing a capacity of 577.1 mAh g−1 at a current density of 300,000 mA g−1. The grasp of catalysis steps within AZIBs can drive solutions beyond state-of-the-art fast-charging batteries. Aqueous Zn-ion batteries are promising devices but their energy storage mechanism remains elusive. Now it is shown that these involve a catalytic mechanism based on water dissociation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


