内侧内叶皮层介导依赖于上下文的间隔计时行为的学习
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
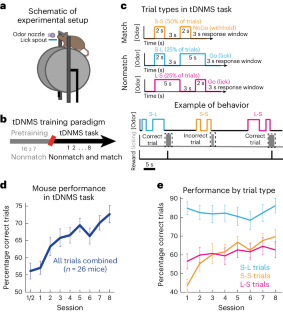
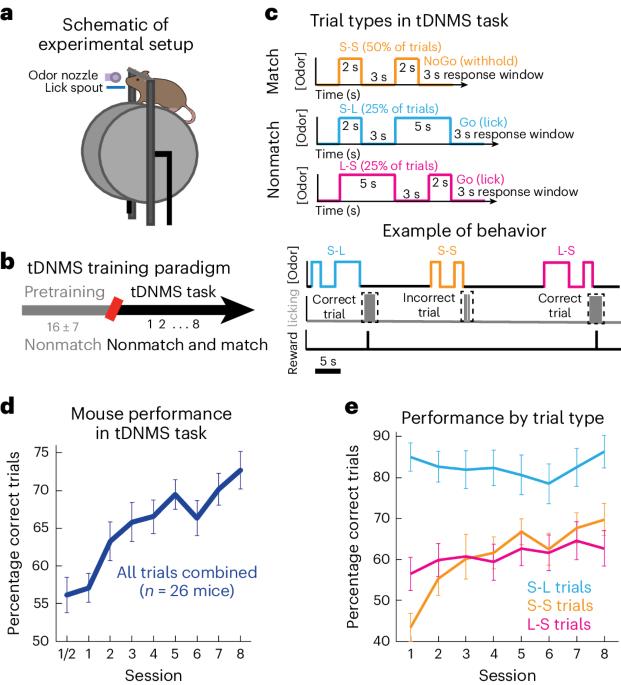
情节记忆需要对经验的时间结构进行编码,并依赖于内侧颞叶的大脑回路,包括内侧内侧皮层(MEC)。最近的研究发现了内侧颞叶皮层的 "时间细胞",这些细胞在完成时间间隔计时任务时会在特定时刻发射信号,共同覆盖整个计时周期。有人推测,MEC时间细胞可提供外显记忆所需的时间信息,但它们是否表现出编码不同时间背景所需的学习动力,目前仍不得而知。为了探索这个问题,我们开发了一种新的行为范式,要求小鼠区分时间情境。结合细胞分辨率钙成像方法,我们发现 MEC 时间细胞显示出与任务学习相关的神经活动。通过化学失活,我们发现 MEC 活动是学习情境依赖性时间间隔行为所必需的。最后,我们发现了一种共同的电路机制,它可以驱动 MEC 中时间细胞和空间选择性神经元的序列活动。我们的研究表明,MEC 时间细胞的时钟样发射可通过学习进行调节,从而跟踪通过经验产生的各种时间结构。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Medial entorhinal cortex mediates learning of context-dependent interval timing behavior
Episodic memory requires encoding the temporal structure of experience and relies on brain circuits in the medial temporal lobe, including the medial entorhinal cortex (MEC). Recent studies have identified MEC ‘time cells’, which fire at specific moments during interval timing tasks, collectively tiling the entire timing period. It has been hypothesized that MEC time cells could provide temporal information necessary for episodic memories, yet it remains unknown whether they display learning dynamics required for encoding different temporal contexts. To explore this, we developed a new behavioral paradigm requiring mice to distinguish temporal contexts. Combined with methods for cellular resolution calcium imaging, we found that MEC time cells display context-dependent neural activity that emerges with task learning. Through chemogenetic inactivation we found that MEC activity is necessary for learning of context-dependent interval timing behavior. Finally, we found evidence of a common circuit mechanism that could drive sequential activity of both time cells and spatially selective neurons in MEC. Our work suggests that the clock-like firing of MEC time cells can be modulated by learning, allowing the tracking of various temporal structures that emerge through experience. The authors examine the role of medial entorhinal cortex (MEC) in learning complex timing behavior. MEC inactivation disrupts task learning, and MEC time cells display context-dependent dynamics that evolve over learning and predict timing behavior.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


