探索介观质量传输对电催化选择性的影响
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
电催化选择性通常是根据活性位点在原子层面上讨论的,而忽略了介观质量输运的更微妙影响。在这里,我们展示了传输如何通过表面结合的反应中间产物在电极和主体电解质之间的交换来控制选择性。我们认为,由此产生的动力学竞争会随着催化剂表面积的变化而变化,并可能与具有重要技术意义的反应相关,例如,在铜基催化剂上电化学还原二氧化碳过程中的不同产物。我们采用多尺度方法,结合微观动力学和输运建模,针对实验文献中的各种展示实例,具体探讨并量化了这种效应。尽管模型简单,但我们的模型在所有介观、微观和原子尺度上正确再现了催化剂粗糙度的选择性趋势。由此产生的洞察力为电催化选择性的变化提供了另一种解释,或者至少是一种补充解释,这些变化被归因于活性位点的纳米结构或掺杂或合金化引起的电子效应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
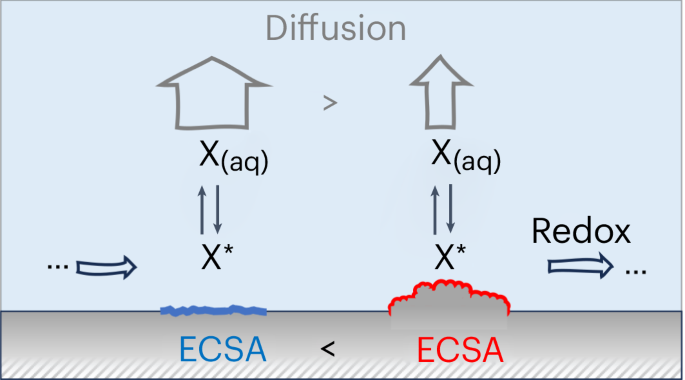
Exploring mesoscopic mass transport effects on electrocatalytic selectivity
Electrocatalytic selectivity is often discussed at the atomic level on the basis of the active site, while ignoring more subtle effects of mesoscopic mass transport. Here we show how transport controls selectivity through the exchange of surface-bound reaction intermediates between the electrode and bulk electrolyte. We argue that the arising kinetic competition changes with the catalyst’s surface area and can become relevant for technologically important reactions including, for example, different products during the electrochemical CO2 reduction on Cu-based catalysts. Combining microkinetic and transport modelling in a multi-scale approach, we specifically explore and quantify this effect for various showcase examples in the experimental literature. Despite its simplicity, our model correctly reproduces selectivity trends with respect to catalyst roughness on all meso-, micro- and atomic scales. The resulting insight provides an alternative or, at least, complementary explanation to changes in electrocatalytic selectivity that have otherwise been attributed to nano-structuring of active sites or electronic effects due to doping or alloying. Mesoscopic mass transport is often ignored but it can influence electrocatalytic processes. This Analysis introduces a simple multi-scale model that couples diffusion to electrochemical surface kinetics and shows how mesoscopic mass transport determines product selectivity through catalyst morphology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


