邻氨基脒氧肟的重氮化用于制备高能 6,5,6-融合 1,2,3-三嗪-3-氧化物。
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
通过邻氨基脒氧肟与亚硝酸钠的反应,设计并合成了两种 6,5,6-融合的 1,2,3-三嗪-3-氧化物(4 和 6)。此外,还分离并鉴定了 1,2,3-三嗪-3-氧化物的开环产物(5、7 和 8)。通过结合理论和实验研究,对开环过程的反应机理进行了全面探索。值得注意的是,化合物 4 表现出值得称道的起爆特性和低敏感性,显示出其作为高能材料的巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
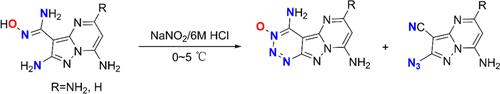
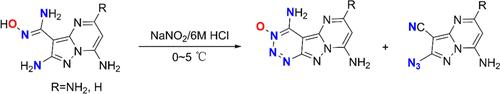
Diazotization of o-Aminoamidoximes for the Preparation of Energetic 6,5,6-Fused 1,2,3-Triazine-3-oxides
Two 6,5,6-fused 1,2,3-triazine-3-oxides (4 and 6) were designed and synthesized via the reaction of o-aminoamidoximes with sodium nitrite. In addition, the ring-opening products (5, 7, and 8) derived from 1,2,3-triazine-3-oxides were isolated and characterized. A comprehensive exploration of the reaction mechanism governing the ring-opening process was performed through a combination of theoretical and experimental studies. Notably, compound 4 exhibited commendable detonation properties and low sensitivity, demonstrating its promising potential as an energetic material.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


