迭代设计揭示超分子肽药共轭物的分子领域关系
IF 5.4
2区 化学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
超分子肽药物共轭物(sPDCs)是通过将药物分子共价连接到一维自组装编程的肽主题上制备而成的,随后这些纤维结构发生物理缠结,从而形成纳米纤维状水凝胶。这类原药材料提供了一个极具吸引力的平台,可利用离散的单组分分子设计实现治疗药物的大规模高效和特定部位给药。然而,sPDC 开发中的一个持续挑战是阐明药物和肽结构域中超分子相互作用之间的关系,以及这些结构域对组装结果和材料特性的影响。在 sPDC 的疏水结构域中与原药一起加入饱和烷基段,可以在一定程度上缓解有序的原药-肽相互作用的必要性。因此,本文制备了九种 sPDC,以反复试验氨基酸序列和疏水性原药-烷基嵌段的设计变量。在生理条件下,所有分子都能自发形成水凝胶,这表明与以前的纯原药设计相比,自组装的热力学路径阻碍较少。然而,对所形成的 sPDC 水凝胶的超分子排列、形成和机械性能以及药物释放曲线进行的材料研究表明,疏水结构域和肽结构域在所形成的组装体的形成和功能中存在复杂的关系。这些结果共同表明,sPDC 材料的特性与整体分子设计有着内在联系,其原药和多肽结构域的耦合作用引导着新材料的特性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
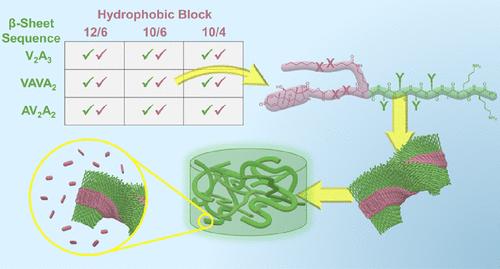
Iterative Design Reveals Molecular Domain Relationships in Supramolecular Peptide–Drug Conjugates
Supramolecular peptide–drug conjugates (sPDCs) are prepared by covalent attachment of a drug moiety to a peptide motif programmed for one-dimensional self-assembly, with subsequent physical entanglement of these fibrillar structures enabling formation of nanofibrous hydrogels. This class of prodrug materials presents an attractive platform for mass-efficient and site-specific delivery of therapeutic agents using a discrete, single-component molecular design. However, a continued challenge in sPDC development is elucidating relationships between supramolecular interactions in their drug and peptide domains and the resultant impacts of these domains on assembly outcomes and material properties. Inclusion of a saturated alkyl segment alongside the prodrug in the hydrophobic domain of sPDCs could relieve some of the necessity for ordered, prodrug–produg interactions. Accordingly, nine sPDCs are prepared here to iterate the design variables of amino acid sequence and hydrophobic prodrug–alkyl block design. All molecules spontaneously formed hydrogels under physiological conditions, indicating a less hindered thermodynamic path to self-assembly relative to previous prodrug-only designs. However, material studies on the supramolecular arrangement, formation, and mechanical properties of the resultant sPDC hydrogels as well as their drug release profiles showed complex relationships between the hydrophobic and peptide domains in the formation and function of the resulting assemblies. Together, these results indicate that sPDC material properties are intrinsically linked to holistic molecular design with coupled contributions from their prodrug and peptide domains in directing properties of the emergent materials.
- Download: Download high-res image (217KB)
- Download: Download full-size image
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomacromolecules
化学-高分子科学
CiteScore
10.60
自引率
4.80%
发文量
417
审稿时长
1.6 months
期刊介绍:
Biomacromolecules is a leading forum for the dissemination of cutting-edge research at the interface of polymer science and biology. Submissions to Biomacromolecules should contain strong elements of innovation in terms of macromolecular design, synthesis and characterization, or in the application of polymer materials to biology and medicine.
Topics covered by Biomacromolecules include, but are not exclusively limited to: sustainable polymers, polymers based on natural and renewable resources, degradable polymers, polymer conjugates, polymeric drugs, polymers in biocatalysis, biomacromolecular assembly, biomimetic polymers, polymer-biomineral hybrids, biomimetic-polymer processing, polymer recycling, bioactive polymer surfaces, original polymer design for biomedical applications such as immunotherapy, drug delivery, gene delivery, antimicrobial applications, diagnostic imaging and biosensing, polymers in tissue engineering and regenerative medicine, polymeric scaffolds and hydrogels for cell culture and delivery.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


