张力激活的碳-碳键
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
机械力驱动着不同的化学反应;然而,其矢量性质导致了与反应轨迹的复杂耦合。在此,我们利用受经典莫尔斯电势及其微分形式启发的物理有机模型,确定有效力常数(keff)和反应能量(ΔE)是支配机械化学动力学的关键分子特征。通过对四种降冰片-2-烯-7-酮(NEO)机械分子进行全面的实验和计算研究,我们确定了这些特征与反应路径上的力依赖性能量变化之间的关系。我们的研究表明,张力键的复杂动力学行为一般可以通过简单的多元线性回归进行定量预测,而多元线性回归是基于两个易于计算的特征,工作流程简单明了。这些结果展示了拉伸力作用下机械化学反应的一般机械框架,并为机械分子设计中的大规模计算筛选提供了一个非常容易获得的工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
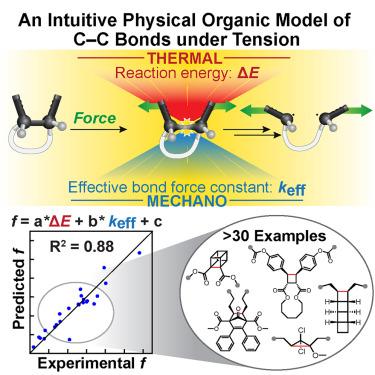
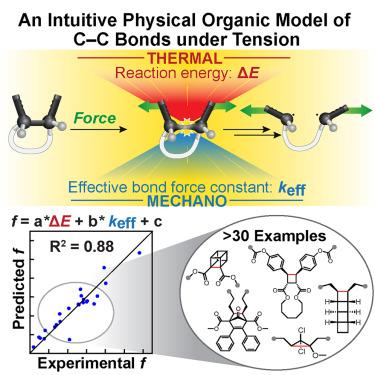
The tension-activated carbon–carbon bond
Mechanical force drives distinct chemical reactions; yet, its vectoral nature results in complicated coupling with reaction trajectories. Here, we utilize a physical organic model inspired by the classical Morse potential and its differential forms to identify effective force constant (keff) and reaction energy (ΔE) as key molecular features that govern mechanochemical kinetics. Through a comprehensive experimental and computational investigation with four norborn-2-en-7-one (NEO) mechanophores, we establish the relationship between these features and the force-dependent energetic changes along the reaction pathways. We show that the complex kinetic behavior of the tensioned bonds is generally and quantitatively predicted by a simple multivariate linear regression based on the two easily computed features with a straightforward workflow. These results demonstrate a general mechanistic framework for mechanochemical reactions under tensile force and provide a highly accessible tool for the large-scale computational screening in the design of mechanophores.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


