PARP抑制剂和免疫检查点抑制剂对胆囊癌有协同疗效
IF 4.5
3区 医学
Q1 GENETICS & HEREDITY
引用次数: 0
摘要
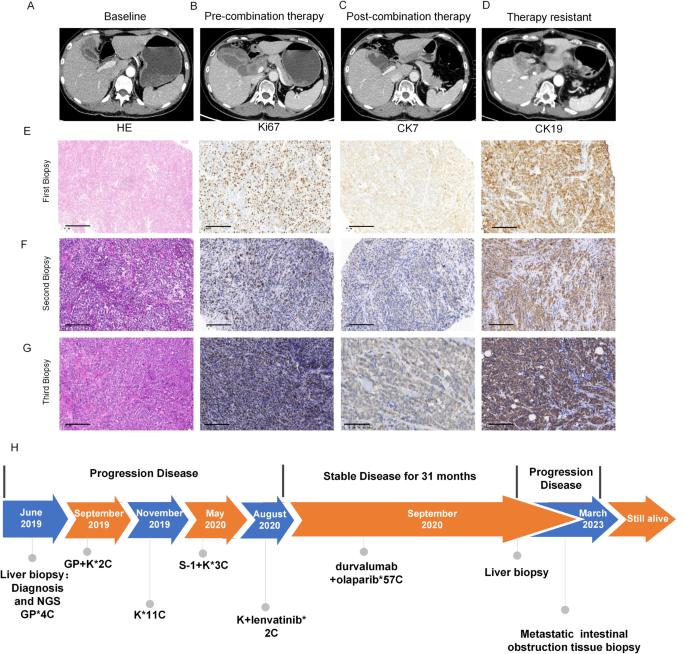
胆囊癌(GBC)是一种侵袭性癌症,预后较差。PARP 抑制剂(PARPi)以 PARP 酶为靶点,对乳腺癌基因(BRCA)突变的患者有疗效。免疫疗法,尤其是免疫检查点抑制剂(ICIs),已经改变了癌症治疗。然而,PARPi 和 ICIs 对 GBC 的综合影响仍不清楚。我们介绍了一例具有突破性意义的病例:一名 BRCA2 突变的 GBC 患者在多线治疗失败后接受了 PARPi 和 ICIs 的联合治疗。下一代测序(NGS-Seq)确定了 BRCA 基因突变。为了进一步研究潜在机制,我们开发了 PARP1-BRCA1-BRCA2 通路相关风险评分(PBscore)系统,通过 RNA-Seq 数据评估 PARPi 对肿瘤免疫微环境的影响。基因表达和功能分析确定了与 PBscore 相关的潜在机制。实验验证在 BRCA 基因野生型或突变患者中使用多重免疫荧光成像和免疫组化技术评估了联合疗法对肿瘤微环境的影响。RNA-Seq分析显示了PBscore、免疫检查点水平、肿瘤浸润免疫细胞(TIIC)和癌症免疫周期之间的相关性。多重免疫荧光成像验证了低PBscore患者可能具有活跃的肿瘤微环境。此外,在耐药后,我们观察到负性免疫检查点(如 CEACAM1)上调,这表明耐药后肿瘤免疫微环境会受到抑制。我们的研究表明,PBscore 可作为预测免疫疗法疗效的生物标记物,为 BRCA2 突变的 GBC 患者提供了一种有希望的选择。本文章由计算机程序翻译,如有差异,请以英文原文为准。
PARP inhibitor and immune checkpoint inhibitor have synergism efficacy in gallbladder cancer
Gallbladder cancer (GBC) is an aggressive cancer with poor prognosis. PARP inhibitors (PARPi) target PARP enzymes and have shown efficacy in patients with breast cancer gene (BRCA) mutations. Immunotherapy, especially immune checkpoint inhibitors (ICIs), has transformed cancer treatment. However, the combined impact of PARPi and ICIs in GBC remains unclear. We present a groundbreaking case of a GBC patient with BRCA2 mutations who received combination therapy with PARPi and ICIs after failing multiple lines of treatment. Next-generation sequencing (NGS-Seq) identified BRCA gene mutations. To further investigate potential mechanisms, we developed a PARP1-BRCA1-BRCA2 pathway-related risk score (PBscore) system to evaluate the impact of PARPi on the tumor immune microenvironment via RNA-Seq data. Gene expression and functional analysis identified potential mechanisms associated with the PBscore. Experimental validation assessed the impact of the combination therapy on the tumor microenvironment using multiplexed immunofluorescence imaging and immunohistochemistry in patients with BRCA gene wild type or mutations. RNA-Seq analysis revealed correlations between PBscore, immune checkpoint levels, tumor-infiltrating immune cells (TIICs), and the cancer-immunity cycle. Multiplexed immunofluorescence imaging validated that low PBscore patients might have an active tumor microenvironment. Furthermore, upon drug resistance, we observed an upregulation of negative immune checkpoints such as CEACAM1, indicating that the tumor immune microenvironment becomes suppressed after resistance. Our study revealed that PBscore could serve as a biomarker to predict immunotherapy efficacy, offering a promising alternative for BRCA2-mutated GBC patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Genes and immunity
医学-免疫学
CiteScore
8.90
自引率
4.00%
发文量
28
审稿时长
6-12 weeks
期刊介绍:
Genes & Immunity emphasizes studies investigating how genetic, genomic and functional variations affect immune cells and the immune system, and associated processes in the regulation of health and disease. It further highlights articles on the transcriptional and posttranslational control of gene products involved in signaling pathways regulating immune cells, and protective and destructive immune responses.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


