淀粉样蛋白病理学血液生物标志物检测的可接受性能--阿尔茨海默病全球首席执行官倡议的建议
IF 28.2
1区 医学
Q1 CLINICAL NEUROLOGY
引用次数: 0
摘要
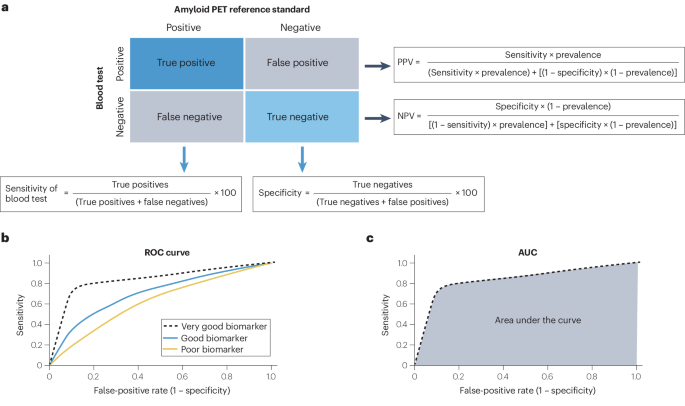
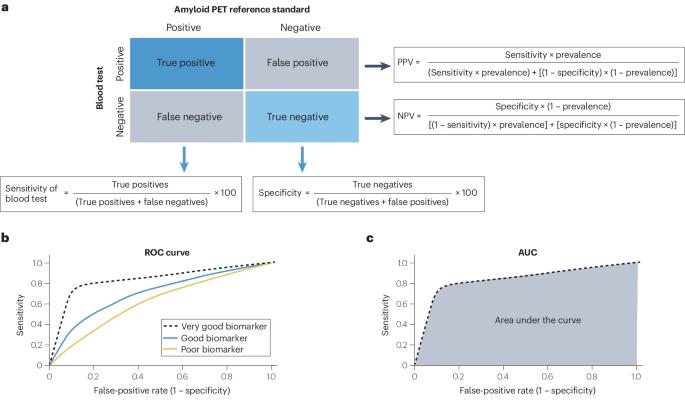
抗淀粉样蛋白治疗早期无症状阿尔茨海默病的方法最近已在一些国家投入临床使用,这大大增加了对淀粉样蛋白病理学生物标志物确认的需求。与淀粉样蛋白 PET 或脑脊液 (CSF) 检测相比,淀粉样蛋白病理学的血液生物标志物 (BBM) 检测更容易接受、更方便、更可扩展,但其性能水平差异很大。阿尔茨海默病全球首席执行官倡议 "召集了一个 BBM 工作组,审议临床使用的 BBM 检测的最低可接受性能。淀粉样蛋白 PET 状态被确定为参考标准。作为淀粉样蛋白 PET 或 CSF 等后续确证检验前的分流检验,BBM 工作组建议 BBM 检验的灵敏度≥90%,特异性≥85%(初级医疗),≥75%-85%(二级医疗),具体取决于后续检验的可用性。如果不进行后续检测,而将 BBM 检测作为确诊检测使用,则其性能应与 CSF 检测相当--灵敏度和特异度约为 90%。重要的是,所有生物标记物检测的预测值都会因检测前淀粉样蛋白病理概率的不同而不同,因此必须在完整的临床背景下进行解释。使用符合这些性能标准的生物标志物检测可使更多人得到准确及时的阿尔茨海默病诊断,并有可能从新疗法中获益。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Acceptable performance of blood biomarker tests of amyloid pathology — recommendations from the Global CEO Initiative on Alzheimer’s Disease
Anti-amyloid treatments for early symptomatic Alzheimer disease have recently become clinically available in some countries, which has greatly increased the need for biomarker confirmation of amyloid pathology. Blood biomarker (BBM) tests for amyloid pathology are more acceptable, accessible and scalable than amyloid PET or cerebrospinal fluid (CSF) tests, but have highly variable levels of performance. The Global CEO Initiative on Alzheimer’s Disease convened a BBM Workgroup to consider the minimum acceptable performance of BBM tests for clinical use. Amyloid PET status was identified as the reference standard. For use as a triaging test before subsequent confirmatory tests such as amyloid PET or CSF tests, the BBM Workgroup recommends that a BBM test has a sensitivity of ≥90% with a specificity of ≥85% in primary care and ≥75–85% in secondary care depending on the availability of follow-up testing. For use as a confirmatory test without follow-up tests, a BBM test should have performance equivalent to that of CSF tests — a sensitivity and specificity of ~90%. Importantly, the predictive values of all biomarker tests vary according to the pre-test probability of amyloid pathology and must be interpreted in the complete clinical context. Use of BBM tests that meet these performance standards could enable more people to receive an accurate and timely Alzheimer disease diagnosis and potentially benefit from new treatments. Anti-amyloid treatments for early symptomatic Alzheimer disease have greatly increased the need for biomarker confirmation of amyloid pathology and blood biomarker tests offer an accessible and scalable biomarker test. This Consensus Statement provides recommendations for the minimum acceptable performance of blood biomarker tests for clinical use.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Neurology
医学-临床神经学
CiteScore
29.90
自引率
0.80%
发文量
138
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Neurology aims to be the premier source of reviews and commentaries for the scientific and clinical communities we serve. We want to provide an unparalleled service to authors, referees, and readers, and we work hard to maximize the usefulness and impact of each article. The journal publishes Research Highlights, Comments, News & Views, Reviews, Consensus Statements, and Perspectives relevant to researchers and clinicians working in the field of neurology. Our broad scope ensures that the work we publish reaches the widest possible audience. Our articles are authoritative, accessible, and enhanced with clearly understandable figures, tables, and other display items. This page gives more detail about the aims and scope of the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


