PRDX6 可提高硒的利用率,限制铁中毒和铁变态反应
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
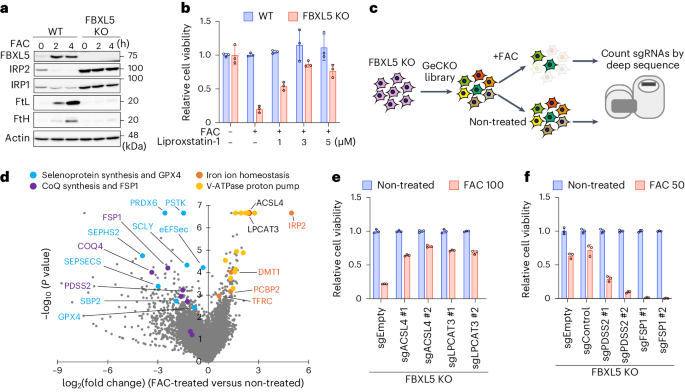
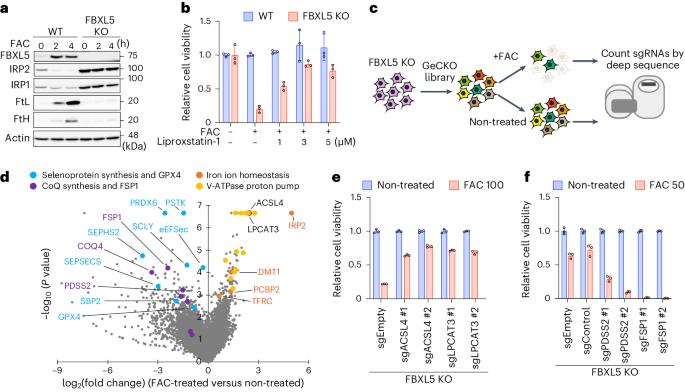
铁卟啉中毒是由铁依赖性脂质氢过氧化物积累诱发的一种调节性细胞死亡。硒蛋白谷胱甘肽过氧化物酶 4(GPX4)通过催化硒半胱氨酸(Sec)残基解毒脂质氢过氧化物,从而抑制铁变态反应。Sec是基因编码的第21个氨基酸,是由其同源的tRNA[Ser]Sec上的活性硒供体生物合成的。在这里,我们发现过氧化物歧化酶 6(PRDX6)是一种新型硒蛋白合成因子。缺失 PRDX6 会降低硒蛋白的表达,并通过 GPX4 的减少诱导铁变态反应。从机理上讲,PRDX6 通过在硒半胱-tRNA[Ser]Sec 合成机制内的蛋白质之间转移硒,提高了细胞内硒的利用效率,从而导致硒半胱-tRNA[Ser]Sec 的高效合成。 这些发现突显了以前未被发现的硒代谢系统,并提供了对铁变态反应的新见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
PRDX6 augments selenium utilization to limit iron toxicity and ferroptosis
Ferroptosis is a form of regulated cell death induced by iron-dependent accumulation of lipid hydroperoxides. Selenoprotein glutathione peroxidase 4 (GPX4) suppresses ferroptosis by detoxifying lipid hydroperoxides via a catalytic selenocysteine (Sec) residue. Sec, the genetically encoded 21st amino acid, is biosynthesized from a reactive selenium donor on its cognate tRNA[Ser]Sec. It is thought that intracellular selenium must be delivered ‘safely’ and ‘efficiently’ by a carrier protein owing to its high reactivity and very low concentrations. Here, we identified peroxiredoxin 6 (PRDX6) as a novel selenoprotein synthesis factor. Loss of PRDX6 decreases the expression of selenoproteins and induces ferroptosis via a reduction in GPX4. Mechanistically, PRDX6 increases the efficiency of intracellular selenium utilization by transferring selenium between proteins within the selenocysteyl-tRNA[Ser]Sec synthesis machinery, leading to efficient synthesis of selenocysteyl-tRNA[Ser]Sec. These findings highlight previously unidentified selenium metabolic systems and provide new insights into ferroptosis. The authors identified PRDX6 as a novel selenoprotein synthesis factor performing an iron-induced ferroptosis screen. They reveal that PRDX6 greatly facilitates selenium utilization for selenoprotein synthesis by acting as a selenide carrier protein.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


