瓜氨酸反应性自身抗体的特殊性、多样性和影响
IF 29.4
1区 医学
Q1 RHEUMATOLOGY
引用次数: 0
摘要
抗瓜氨酸蛋白抗体(ACPA)作为类风湿性关节炎的高度特异性血清生物标志物,自 25 年前进入临床以来,一直是广泛研究的课题。这种标志性的 B 细胞反应在发病前数年就已出现,显示出患者间自身抗原的差异性,并与不良的临床预后有关。技术和科学的进步揭示了克隆的广泛多样性和有趣的特征,包括高水平的体细胞超突变、可变域 N-连接糖基化、类合肽相互作用以及克隆对瓜氨酸化、氨甲酰化和乙酰化表位的特异性多反应性。在血液循环和组织中发现的 ACPAs 有不同的同型和亚型,并由浆细胞和长寿命浆细胞分泌。值得注意的是,尽管有报道称某些单克隆 ACPAs 具有促发疾病的特征,但现在的研究结果表明,某些单克隆 ACPAs 可在小鼠模型中治疗性地阻断关节炎和炎症。利用源自患者的多克隆和单克隆抗体进行的大量功能性研究为 ACPA 在关节炎中的致病和保护作用提供了证据。要了解 ACPA 的作用,需要结合类风湿性关节炎 B 细胞生物学、环境诱因和慢性抗原暴露等不同方面来考虑其免疫学特性。新出现的情况表明瓜氨酸反应性自身抗体发挥着复杂的作用,其中抗体克隆的多样性和动态性可决定临床进展和表现。本文章由计算机程序翻译,如有差异,请以英文原文为准。
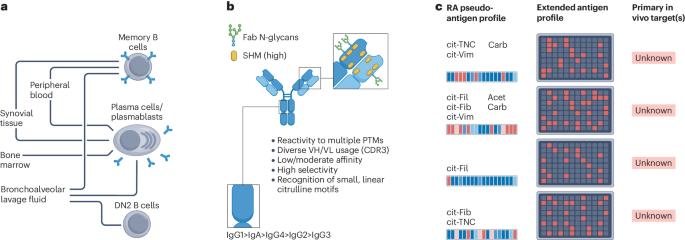
The peculiar features, diversity and impact of citrulline-reactive autoantibodies
Since entering the stage 25 years ago as a highly specific serological biomarker for rheumatoid arthritis, anti-citrullinated protein antibodies (ACPAs) have been a topic of extensive research. This hallmark B cell response arises years before disease onset, displays interpatient autoantigen variability, and is associated with poor clinical outcomes. Technological and scientific advances have revealed broad clonal diversity and intriguing features including high levels of somatic hypermutation, variable-domain N-linked glycosylation, hapten-like peptide interactions, and clone-specific multireactivity to citrullinated, carbamylated and acetylated epitopes. ACPAs have been found in different isotypes and subclasses, in both circulation and tissue, and are secreted by both plasmablasts and long-lived plasma cells. Notably, although some disease-promoting features have been reported, results now demonstrate that certain monoclonal ACPAs therapeutically block arthritis and inflammation in mouse models. A wealth of functional studies using patient-derived polyclonal and monoclonal antibodies have provided evidence for pathogenic and protective effects of ACPAs in the context of arthritis. To understand the roles of ACPAs, one needs to consider their immunological properties by incorporating different facets such as rheumatoid arthritis B cell biology, environmental triggers and chronic antigen exposure. The emerging picture points to a complex role of citrulline-reactive autoantibodies, in which the diversity and dynamics of antibody clones could determine clinical progression and manifestations. In this Review, the authors provide an overview of the immunological, clinical and pathophysiological features of anti-citrullinated protein antibodies in rheumatoid arthritis, highlighting the latest findings regarding the complex contribution of anti-citrullinated protein antibodies to the disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Rheumatology
医学-风湿病学
CiteScore
29.90
自引率
0.90%
发文量
137
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Rheumatology is part of the Nature Reviews portfolio of journals. The journal scope covers the entire spectrum of rheumatology research. We ensure that our articles are accessible to the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


