少突胶质细胞的发育起源决定其在成人大脑中的功能
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
在小鼠胚胎前脑中,发育不同的少突胶质细胞祖细胞群及其后代少突胶质细胞从三个不同的区域出现,其时空梯度从腹侧到背侧。然而,这种少突胶质细胞发育异质性的功能重要性尚不清楚。利用基因策略消减背侧来源的少突胶质细胞系细胞(OLCs),我们在本文中展示了成年中枢神经系统中背侧来源的 OLCs 通常所在的区域会被腹侧来源的 OLCs 所填充和髓鞘化。这些异位少突胶质细胞(eOLs)具有独特的基因表达谱以及细微的髓鞘化异常。异位少突胶质细胞无法完全承担原始背侧来源细胞的作用,导致成年动物出现运动和认知障碍。这项研究揭示了少突胶质细胞系内发育异质性的重要性及其对大脑平衡功能的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
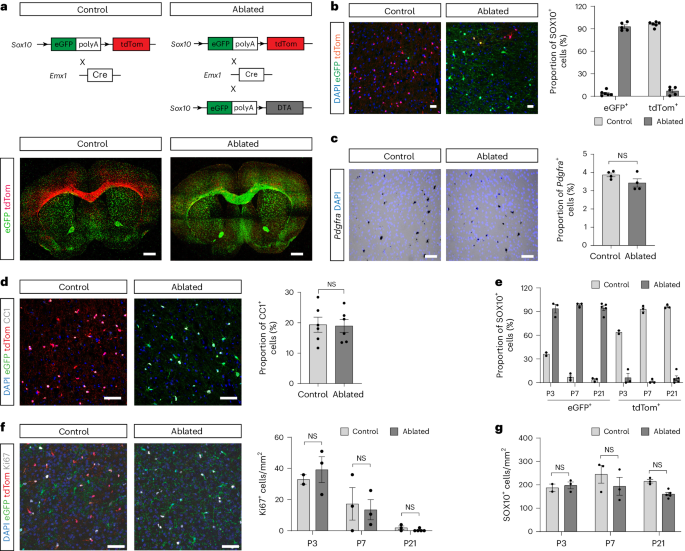
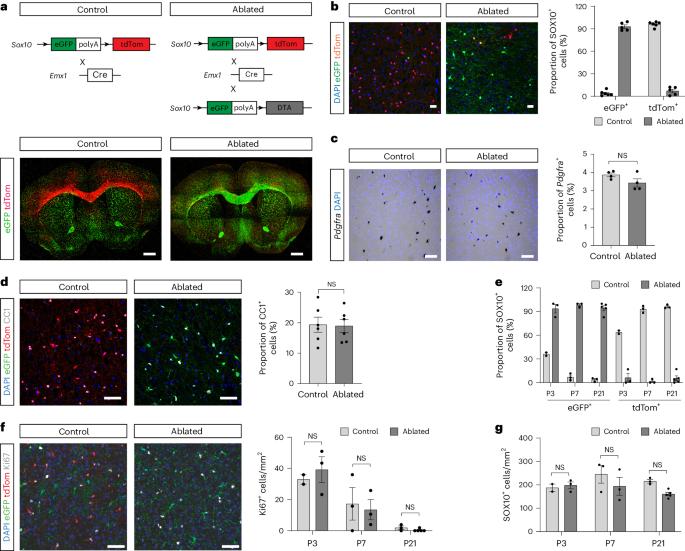
Developmental origin of oligodendrocytes determines their function in the adult brain
In the mouse embryonic forebrain, developmentally distinct oligodendrocyte progenitor cell populations and their progeny, oligodendrocytes, emerge from three distinct regions in a spatiotemporal gradient from ventral to dorsal. However, the functional importance of this oligodendrocyte developmental heterogeneity is unknown. Using a genetic strategy to ablate dorsally derived oligodendrocyte lineage cells (OLCs), we show here that the areas in which dorsally derived OLCs normally reside in the adult central nervous system become populated and myelinated by OLCs of ventral origin. These ectopic oligodendrocytes (eOLs) have a distinctive gene expression profile as well as subtle myelination abnormalities. The failure of eOLs to fully assume the role of the original dorsally derived cells results in locomotor and cognitive deficits in the adult animal. This study reveals the importance of developmental heterogeneity within the oligodendrocyte lineage and its importance for homeostatic brain function. Here the authors show that ventrally derived oligodendrocytes (OLs) can myelinate areas usually populated by dorsally derived OLs but cannot functionally compensate, as animals populated only by ventrally derived OLs show locomotor and cognitive deficits.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


