在骨关节炎发展过程中,GYY4137诱导的p65硫酸化作用可通过改善线粒体功能保护滑膜巨噬细胞免受脓毒症的侵袭。
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
导言:骨关节炎(OA)是最常见的关节炎,其特点是滑膜炎症和关节软骨的逐渐丧失。尽管 GYY4137 是一种新型的缓释硫化氢(H2S)供体,具有强效抗炎特性,可调节 OA 的进展,但其潜在机制仍不清楚:在这项研究中,我们验证了 GYY4137 对 OA 病理过程的保护作用,并阐明了其潜在的调控机制:方法:在原代小鼠软骨细胞或小鼠巨噬细胞系 raw 264.7 中进行细胞转染、免疫荧光染色、EdU 检测、透射电子显微镜、线粒体膜电位测量、电泳迁移检测、硫水化检测、qPCR 和 Western 印迹检测等体外研究。体内研究采用了 DMM 诱导的 OA 小鼠模型和 C57BL/6 背景的巨噬细胞特异性 p65 基因敲除(p65f/f LysM-CreERT2)小鼠:结果:我们发现,GYY4137能通过抑制滑膜脓毒症减轻OA的进展。此外,我们的体外研究数据显示,GYY4137 可减轻炎症诱导的 NLRP3 和 caspase-1 激活,并减少巨噬细胞中 IL-1β 的产生。从机理上讲,GYY4137 在炎症刺激下增加了 NF-kB p65 的过硫化,从而减少了细胞活性氧(ROS)的积累,改善了线粒体功能障碍。通过定点突变,我们发现 H2S 会使 p65 蛋白中的半胱氨酸 38 发生过硫化,从而阻碍 p65 的转录活性,p65 突变体会削弱巨噬细胞对 GYY4137 的反应:这些发现提示了一种机制,即 GYY4137 通过对 p65 的氧化还原修饰参与抑制 OA 激活 NLRP3,从而调节炎症反应。因此,我们认为 GYY4137 是一种治疗 OA 的新型治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
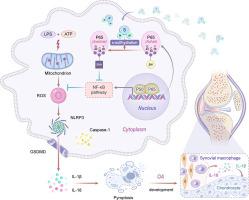

GYY4137-induced p65 sulfhydration protects synovial macrophages against pyroptosis by improving mitochondrial function in osteoarthritis development
Introduction
Osteoarthritis (OA) is the most common arthritis that is characterized by the progressive synovial inflammation and loss of articular cartilage. Although GYY4137 is a novel and slow-releasing hydrogen sulfide (H2S) donor with potent anti-inflammatory properties that may modulate the progression of OA, its underlying mechanism remains unclear.
Objectives
In this study, we validated the protective role of GYY4137 against OA pathological courses and elucidated its underlying regulatory mechanisms.
Methods
Cell transfection, immunofluorescence staining, EdU assay, transmission electron microscopy, mitochondrial membrane potential measurement, electrophoretic mobility shift assay, sulfhydration assay, qPCR and western blot assays were performed in the primary mouse chondrocytes or the mouse macrophage cell line raw 264.7 for in vitro study. DMM-induced OA mice model and Macrophage-specific p65 knockout (p65f/f LysM-CreERT2) mice on the C57BL/6 background were used for in vivo study.
Results
We found that GYY4137 can alleviate OA progress by suppressing synovium pyroptosis in vivo. Moreover, our in vitro data revealed that GYY4137 attenuates inflammation-induced NLRP3 and caspase-1 activation and results in a decrease of IL-1β production in macrophages. Mechanistically, GYY4137 increased persulfidation of NF-kB p65 in response to inflammatory stimuli that results in a decrease of cellular reactive oxygen species (ROS) accumulation and ameliorates mitochondrial dysfunctions. Using site-directed mutagenesis, we showed that H2S persulfidates cysteine38 in p65 protein and hampers p65 transcriptional activity, and p65 mutant impaired macrophage responses to GYY4137.
Conclusion
These findings suggest a mechanism by which GYY4137 through redox modification of p65 participates in inhibiting NLRP3 activation by OA to regulate inflammatory responses. Thus, we propose that GYY4137 represents a promising novel therapeutic strategy for the treatment of OA.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


