分辨率为 1.9 Å 的人类 80S 核糖体结构揭示了 RNA 中化学修饰和离子的分子作用
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
人类蛋白质合成机制的核糖体 RNA 包含在核糖体生物发生过程中引入的大量化学修饰。在这里,我们展示了 1.9 Å 分辨率的人类 80S 核糖体低温电子显微镜结构,该结构解析了大量新的核糖体 RNA 修饰以及 Zn2+、K+ 和 Mg2+ 等重要功能离子,包括与之相关的单个水分子。质谱法证实了 2′-O-甲基化、假尿苷和碱基修饰,从而对 230 个位点进行了全面研究,其中许多位点以前无法解决。它们编排了 RNA 内部以及与蛋白质界面(包括完全组装的 80S 核糖体的核糖体亚基界面)的关键相互作用。尿苷酸异构化被证明是一般 RNA 中 U-A 碱基对稳定的关键机制。化学修饰和离子的结构环境对成熟人类核糖体的 RNA 结构至关重要,因此提供了一个结构框架来解决它们在健康状态和人类疾病中的作用问题。本文章由计算机程序翻译,如有差异,请以英文原文为准。
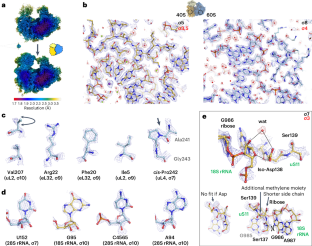
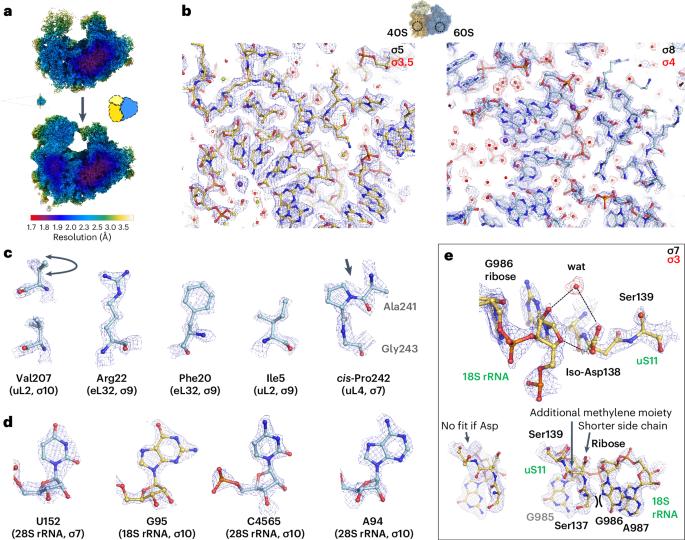
The structure of the human 80S ribosome at 1.9 Å resolution reveals the molecular role of chemical modifications and ions in RNA
The ribosomal RNA of the human protein synthesis machinery comprises numerous chemical modifications that are introduced during ribosome biogenesis. Here we present the 1.9 Å resolution cryo electron microscopy structure of the 80S human ribosome resolving numerous new ribosomal RNA modifications and functionally important ions such as Zn2+, K+ and Mg2+, including their associated individual water molecules. The 2′-O-methylation, pseudo-uridine and base modifications were confirmed by mass spectrometry, resulting in a complete investigation of the >230 sites, many of which could not be addressed previously. They choreograph key interactions within the RNA and at the interface with proteins, including at the ribosomal subunit interfaces of the fully assembled 80S ribosome. Uridine isomerization turns out to be a key mechanism for U–A base pair stabilization in RNA in general. The structural environment of chemical modifications and ions is primordial for the RNA architecture of the mature human ribosome, hence providing a structural framework to address their role in healthy states and in human diseases. The cryo-EM structure of the full 80S human ribosome is presented at 1.9 Å resolution. Numerous new chemical modifications are resolved, resulting in over 230 annotated sites cross-validated by mass spectometry. Ions and water molecules are seen to stabilize the RNA architecture.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


