与年龄和新陈代谢相关的肠道微生物组特征差异调节心血管疾病风险
IF 50
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
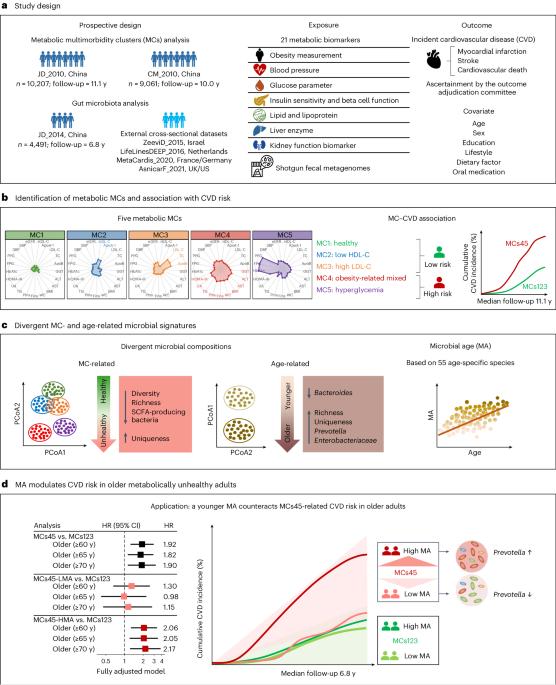
深入了解肠道微生物组与新陈代谢和衰老之间的关系,对于调整干预措施以促进健康长寿至关重要。在一个由 10207 名 40-93 岁个体组成的发现队列中,我们使用 21 个代谢参数将个体分为五个群组,称为代谢多病群组(MC),代表不同的代谢亚型。与代谢健康群组(MC1)相比,"肥胖相关混合 "群组(MC4)和 "高血糖 "群组(MC5)的11.1年心血管疾病(CVD)风险分别增加了75%(多变量调整危险比(HR):1.75,95%置信区间(CI):1.43-2.14)和117%(2.17,1.72-2.74)。这些关联在第二个队列中得到了验证,该队列共有 9,061 人,随访时间为 10.0 年。根据对发现队列中 4,491 个猎枪粪便元基因组的分析,我们发现肠道微生物组成与 MCs 和年龄都有关联。接下来,我们利用 55 种年龄特异性微生物物种来捕捉生物年龄,从而制定了肠道微生物年龄(MA)指标,并在由 4425 份元基因组样本组成的四个外部队列中进行了验证。在 60 岁或以上的人群中,与 MC1、MC2 或 MC3 相比,与 MC4 或 MC5 相关的心血管疾病风险增加在高 MA 值人群中会加剧,但在低 MA 值人群中会降低,这与年龄、性别以及其他生活方式和饮食因素无关。在这种模式中,较年轻的MA似乎可以抵消代谢功能障碍导致的心血管疾病风险,这意味着MA对代谢不健康的老年人的心血管健康具有调节作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
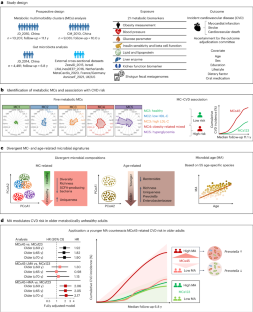
Divergent age-associated and metabolism-associated gut microbiome signatures modulate cardiovascular disease risk
Insight into associations between the gut microbiome with metabolism and aging is crucial for tailoring interventions to promote healthy longevity. In a discovery cohort of 10,207 individuals aged 40–93 years, we used 21 metabolic parameters to classify individuals into five clusters, termed metabolic multimorbidity clusters (MCs), that represent different metabolic subphenotypes. Compared to the cluster classified as metabolically healthy (MC1), clusters classified as ‘obesity-related mixed’ (MC4) and ‘hyperglycemia’ (MC5) exhibited an increased 11.1-year cardiovascular disease (CVD) risk by 75% (multivariable-adjusted hazard ratio (HR): 1.75, 95% confidence interval (CI): 1.43–2.14) and by 117% (2.17, 1.72–2.74), respectively. These associations were replicated in a second cohort of 9,061 individuals with a 10.0-year follow-up. Based on analysis of 4,491 shotgun fecal metagenomes from the discovery cohort, we found that gut microbial composition was associated with both MCs and age. Next, using 55 age-specific microbial species to capture biological age, we developed a gut microbial age (MA) metric, which was validated in four external cohorts comprising 4,425 metagenomic samples. Among individuals aged 60 years or older, the increased CVD risk associated with MC4 or MC5, as compared to MC1, MC2 or MC3, was exacerbated in individuals with high MA but diminished in individuals with low MA, independent of age, sex and other lifestyle and dietary factors. This pattern, in which younger MA appears to counteract the CVD risk attributable to metabolic dysfunction, implies a modulating role of MA in cardiovascular health for metabolically unhealthy older people. Data from two large longitudinal cohorts in China, in which the participants were clustered into five groups based on their metabolic characteristics, show that a signature of microbiome age modulates the risk of cardiovascular disease in metabolically unhealthy individuals.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


