ATP酶-核酸酶双酶抗噬菌体防御复合物的分子和结构基础。
IF 28.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
在细菌免疫反应中,将不同的酶效应器耦合在一起是抵御噬菌体感染的有效策略,例如被广泛研究的 III 型 CRISPR-Cas 系统中的核酸酶和环化酶活动。然而,人们对其他细菌防御系统中的协同酶活性知之甚少。在这里,我们从生物化学和结构上描述了双组分防御系统 DUF4297-HerA,证明 DUF4297-HerA 通过协同裂解 dsDNA 和水解 ATP 来抵抗噬菌体感染。DUF4297 单独形成二聚体,而 HerA 单独以非平面分裂螺旋六聚体的形式存在,两者都表现出极低的酶活性。有趣的是,DUF4297 和 HerA 组装成一个约 1 MDa 的超分子复合物,其中两层 DUF4297(每层 6 个 DUF4297 分子)通过相邻 DUF4297 分子的层间二聚化连接在一起,堆叠在 HerA 六聚体的顶部。重要的是,复合体的组装促进了上层 DUF4297 分子的二聚化,并使 HerA 从非平面六聚体转变为平面六聚体,从而激活了它们各自的酶活性,以抑制噬菌体感染。总之,我们的研究结果不仅描述了一种新型双酶抗噬菌体防御系统的特征,而且揭示了细菌免疫中通过合作复合物组装的独特激活机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
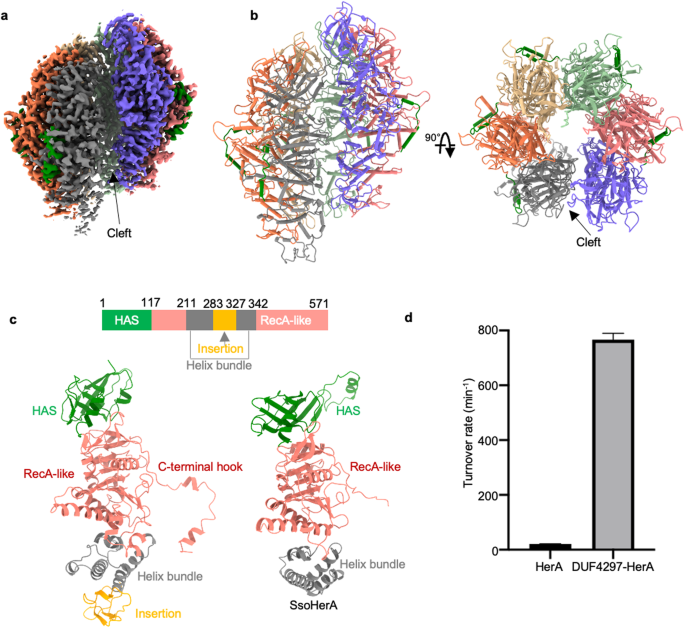
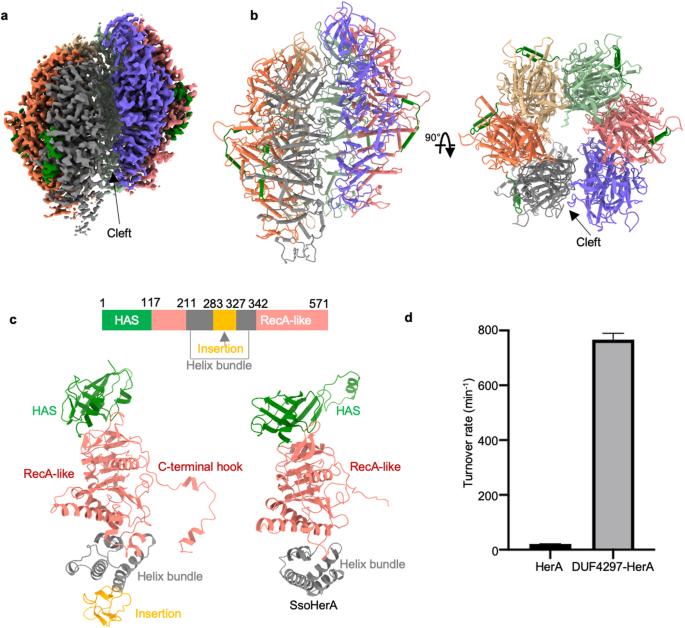
Molecular and structural basis of an ATPase-nuclease dual-enzyme anti-phage defense complex
Coupling distinct enzymatic effectors emerges as an efficient strategy for defense against phage infection in bacterial immune responses, such as the widely studied nuclease and cyclase activities in the type III CRISPR-Cas system. However, concerted enzymatic activities in other bacterial defense systems are poorly understood. Here, we biochemically and structurally characterize a two-component defense system DUF4297–HerA, demonstrating that DUF4297–HerA confers resistance against phage infection by cooperatively cleaving dsDNA and hydrolyzing ATP. DUF4297 alone forms a dimer, and HerA alone exists as a nonplanar split spiral hexamer, both of which exhibit extremely low enzymatic activity. Interestingly, DUF4297 and HerA assemble into an approximately 1 MDa supramolecular complex, where two layers of DUF4297 (6 DUF4297 molecules per layer) linked via inter-layer dimerization of neighboring DUF4297 molecules are stacked on top of the HerA hexamer. Importantly, the complex assembly promotes dimerization of DUF4297 molecules in the upper layer and enables a transition of HerA from a nonplanar hexamer to a planar hexamer, thus activating their respective enzymatic activities to abrogate phage infection. Together, our findings not only characterize a novel dual-enzyme anti-phage defense system, but also reveal a unique activation mechanism by cooperative complex assembly in bacterial immunity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Research
生物-细胞生物学
CiteScore
53.90
自引率
0.70%
发文量
2420
审稿时长
2.3 months
期刊介绍:
Cell Research (CR) is an international journal published by Springer Nature in partnership with the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences (CAS). It focuses on publishing original research articles and reviews in various areas of life sciences, particularly those related to molecular and cell biology. The journal covers a broad range of topics including cell growth, differentiation, and apoptosis; signal transduction; stem cell biology and development; chromatin, epigenetics, and transcription; RNA biology; structural and molecular biology; cancer biology and metabolism; immunity and molecular pathogenesis; molecular and cellular neuroscience; plant molecular and cell biology; and omics, system biology, and synthetic biology. CR is recognized as China's best international journal in life sciences and is part of Springer Nature's prestigious family of Molecular Cell Biology journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


