AMPA 受体中的异位竞争和抑制作用
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
兴奋性神经传递主要由α-氨基-3-羟基-5-甲基-4-异恶唑丙酸(AMPA)亚型离子型谷氨酸受体(AMPARs)介导。负性异位调节剂是抑制 AMPAR 激活的候选治疗药物,可与正性调节剂竞争,通过尚未解决的机制控制 AMPAR 的功能。在这里,我们展示了异位抑制将 AMPAR 推入一种独特的状态,这种状态既能阻止激活,也能阻止正性异位调节。我们使用低温电子显微镜捕获了与谷氨酸结合的 AMPARs,同时负性异位调节剂 GYKI-52466 和正性异位调节剂环噻嗪竞争 AMPARs 的控制权。GYKI-52466 与离子通道颈部结合,通过使配体结合域与离子通道脱钩来抑制 AMPARs。配体结合结构域的重新排列使环噻嗪位点断裂,从而阻止了正向调节。我们的数据为了解 AMPARs 的异构性以及合理设计针对神经系统疾病的 AMPARs 治疗方法提供了一个框架。本文章由计算机程序翻译,如有差异,请以英文原文为准。
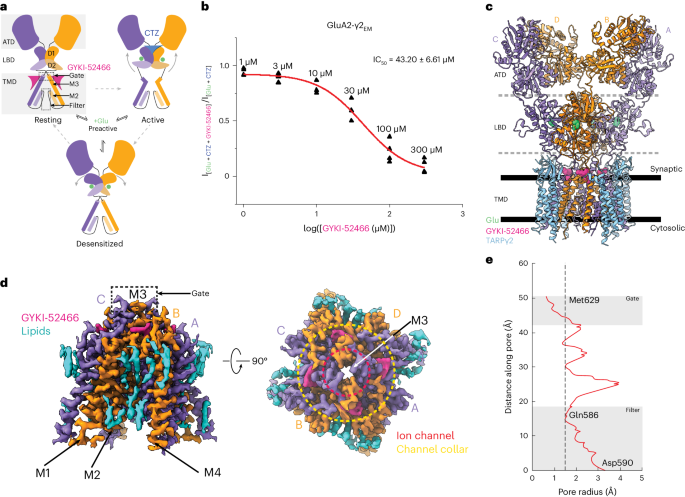
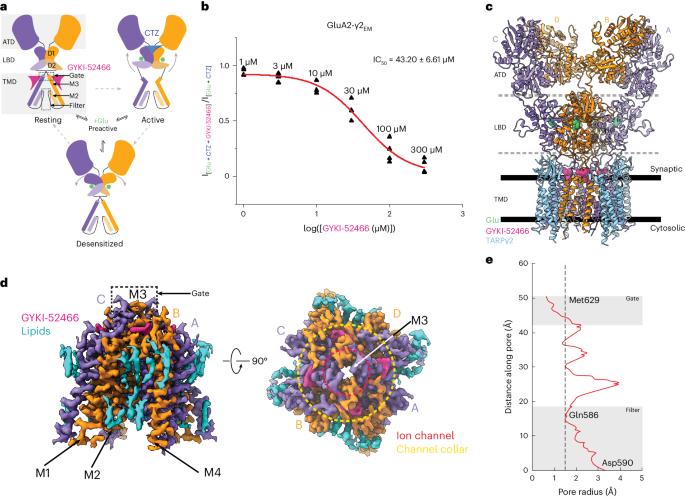
Allosteric competition and inhibition in AMPA receptors
Excitatory neurotransmission is principally mediated by α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-subtype ionotropic glutamate receptors (AMPARs). Negative allosteric modulators are therapeutic candidates that inhibit AMPAR activation and can compete with positive modulators to control AMPAR function through unresolved mechanisms. Here we show that allosteric inhibition pushes AMPARs into a distinct state that prevents both activation and positive allosteric modulation. We used cryo-electron microscopy to capture AMPARs bound to glutamate, while a negative allosteric modulator, GYKI-52466, and positive allosteric modulator, cyclothiazide, compete for control of the AMPARs. GYKI-52466 binds in the ion channel collar and inhibits AMPARs by decoupling the ligand-binding domains from the ion channel. The rearrangement of the ligand-binding domains ruptures the cyclothiazide site, preventing positive modulation. Our data provide a framework for understanding allostery of AMPARs and for rational design of therapeutics targeting AMPARs in neurological diseases. Using cryo-electron microscopy, the authors reveal the mechanism by which perampanel-like molecules inhibit AMPA receptors. They show that the inhibitors decouple the ligand-binding domain from the ion channel after neurotransmitter binding and outcompete positive modulators.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


