Claudin18.2 特异性 CAR T 细胞在胃肠道癌症中的应用:1 期试验最终结果。
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
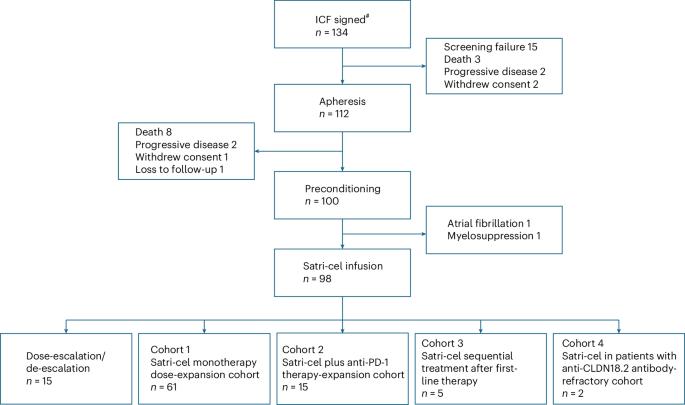
Claudin18.2(CLDN18.2)在各种恶性肿瘤尤其是胃肠道癌症的发病过程中高度表达,正在成为癌症治疗的新靶点。Satricabtagene autoleucel(satri-cel)/CT041是一种靶向CLDN18.2的自体嵌合抗原受体(CAR)T细胞,CT041-CG4006试验的中期结果已于2022年6月报道。在此,我们介绍这项单臂、开放标签、1期试验的最终结果,该试验评估了satri-cel在CLDN18.2阳性晚期胃肠道癌症患者中的安全性和有效性。该试验包括剂量递增阶段(n = 15)和剂量扩大阶段,共分为四个不同组群(n = 83):队列 1:61 例标准化疗难治性胃肠道癌症患者接受 satri-cel 单药治疗;队列 2:15 例标准化疗难治性胃肠道癌症患者接受 satri-cel 加抗 PD-1 治疗;队列 3:5 例胃肠道癌症患者接受 satri-cel 作为一线治疗后的序贯治疗;队列 4:2 例抗CLDN18.2 单克隆抗体难治性胃肠道癌症患者接受 satri-cel 单药治疗。2 单克隆抗体难治性胃癌患者的 satri-cel 单药治疗。主要终点是安全性,次要终点包括疗效、药代动力学和免疫原性。共有98名患者接受了饱和凝胶输注,其中89人使用了2.5 × 108、6人使用了3.75 × 108、3人使用了5.0 × 108 CAR T细胞。中位随访时间为注射后 32.4 个月(95% 置信区间 (CI):27.3 至 36.5)。未报告剂量限制性毒性、治疗相关死亡或免疫效应细胞相关神经毒性综合征。96.9%的患者出现细胞因子释放综合征,均为1-2级。8例(8.2%)患者出现胃黏膜损伤。所有98名患者的总反应率和疾病控制率分别为38.8%和91.8%,无进展生存期和总生存期的中位数分别为4.4个月(95% CI:3.7,6.6)和8.8个月(95% CI:7.1,10.2)。Satri-cel对CLDN18.2阳性晚期胃肠癌患者具有治疗潜力和可控的安全性。ClinicalTrials.gov identifier:NCT03874897 。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial final results
Claudin18.2 (CLDN18.2) is highly expressed with the development of various malignant tumors, especially gastrointestinal cancers, and is emerging as a new target for cancer treatment. Satricabtagene autoleucel (satri-cel)/CT041 is an autologous chimeric antigen receptor (CAR) T cell targeting CLDN18.2, and the interim results of the CT041-CG4006 trial were reported in June 2022. Here we present the final results of this single-arm, open-label, phase 1 trial, which evaluated the safety and efficacy of satri-cel in patients with CLDN18.2-positive advanced gastrointestinal cancers. This trial included a dose-escalation stage (n = 15) and a dose-expansion stage in four different cohorts (total n = 83): cohort 1, satri-cel monotherapy in 61 patients with standard chemotherapy-refractory gastrointestinal cancers; cohort 2, satri-cel plus anti-PD-1 therapy in 15 patients with standard chemotherapy-refractory gastrointestinal cancers; cohort 3, satri-cel as sequential treatment after first-line therapy in five patients with gastrointestinal cancers; and cohort 4, satri-cel monotherapy in two patients with anti-CLDN18.2 monoclonal antibody-refractory gastric cancer. The primary endpoint was safety; secondary endpoints included efficacy, pharmacokinetics and immunogenicity. A total of 98 patients received satri-cel infusion, among whom 89 were dosed with 2.5 × 108, six with 3.75 × 108 and three with 5.0 × 108 CAR T cells. Median follow-up was 32.4 months (95% confidence interval (CI): 27.3, 36.5) since apheresis. No dose-limiting toxicities, treatment-related deaths or immune effector cell-associated neurotoxicity syndrome were reported. Cytokine release syndrome occurred in 96.9% of patients, all classified as grade 1–2. Gastric mucosal injuries were identified in eight (8.2%) patients. The overall response rate and disease control rate in all 98 patients were 38.8% and 91.8%, respectively, and the median progression-free survival and overall survival were 4.4 months (95% CI: 3.7, 6.6) and 8.8 months (95% CI: 7.1, 10.2), respectively. Satri-cel demonstrates therapeutic potential with a manageable safety profile in patients with CLDN18.2-positive advanced gastrointestinal cancer. ClinicalTrials.gov identifier: NCT03874897 . In the largest study so far of CAR T cells in patients with solid tumors, autologous CLDN18.2-targeting CAR T cell therapy was well tolerated in patients with advanced CLDN18.2-positive gastrointestinal cancers, with an overall response rate of 42.2% and a disease control rate of 91.1%.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


