用于检测前列腺癌的风险计算器:系统综述。
IF 5.1
2区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
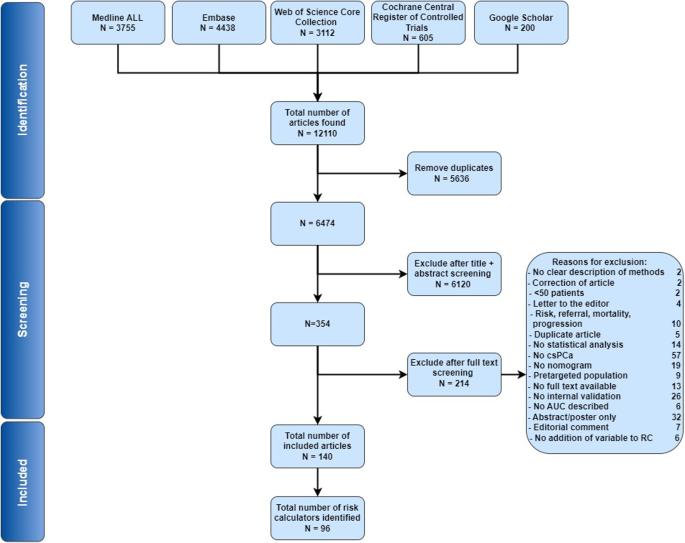
背景:前列腺癌(PCa)(早期)检测带来了巨大挑战,包括不必要的检测和潜在的过度诊断风险。因此,欧洲泌尿外科协会建议采用个体风险适应方法,将风险计算器(RC)纳入 PCa 检测途径。在 "欧盟筛查前列腺癌意识和倡议"(PRAISE-U)项目(https://uroweb.org/praise-u)的背景下,我们旨在概述目前适用于早期 PCa 检测算法的临床 RC:我们进行了一项系统性回顾,以确定预测活检时发现有临床意义的 PCa 的 RC。我们在 Medline ALL、Embase、Web of Science Core Collection、Cochrane Central Register of Controlled Trials 和 Google Scholar 等数据库中检索了 2010 年 1 月至 2023 年 7 月期间的出版物。我们以 "前列腺癌"、"筛查/诊断 "和 "预测模型 "为关键词检索了相关文献。纳入标准包括系统综述、荟萃分析和临床试验。排除标准适用于涉及预先锁定的高危人群、已确诊的 PCa 患者或样本量少于 50 名男性的研究:我们确定了 6474 篇文章,在筛选摘要和全文后,纳入了其中的 140 篇。我们总共发现了 96 篇独特的 RC。其中,45 项经过外部验证,28 项在多个队列中得到验证。在外部验证的 RC 中,17 个基于临床因素,19 个结合了临床因素和 MRI 详情,4 个单独基于血液生物标记物或与临床因素相结合,5 个包括尿液生物标记物。外部验证的 RC 的中位 AUC 在 0.63 到 0.93 之间:本系统综述对目前可用的 RC、其不同的使用情况以及在验证队列中的表现进行了广泛的分析。RC 不断证明其有能力减轻与早期检测相关的局限性,并已被纳入现代实践和筛查试验中。然而,外部验证数据的缺乏引起了人们对许多 RC 的担忧,在评估特定 RC 是否适用于目标人群时,必须考虑到这一疏漏。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Risk calculators for the detection of prostate cancer: a systematic review
Prostate cancer (PCa) (early) detection poses significant challenges, including unnecessary testing and the risk of potential overdiagnosis. The European Association of Urology therefore suggests an individual risk-adapted approach, incorporating risk calculators (RCs) into the PCa detection pathway. In the context of ‘The PRostate Cancer Awareness and Initiative for Screening in the European Union’ (PRAISE-U) project ( https://uroweb.org/praise-u ), we aim to provide an overview of the currently available clinical RCs applicable in an early PCa detection algorithm. We performed a systematic review to identify RCs predicting detection of clinically significant PCa at biopsy. A search was performed in the databases Medline ALL, Embase, Web of Science Core Collection, Cochrane Central Register of Controlled Trials and Google Scholar for publications between January 2010 and July 2023. We retrieved relevant literature by using the terms “prostate cancer”, “screening/diagnosis” and “predictive model”. Inclusion criteria included systematic reviews, meta-analyses, and clinical trials. Exclusion criteria applied to studies involving pre-targeted high-risk populations, diagnosed PCa patients, or a sample sizes under 50 men. We identified 6474 articles, of which 140 were included after screening abstracts and full texts. In total, we identified 96 unique RCs. Among these, 45 underwent external validation, with 28 validated in multiple cohorts. Of the externally validated RCs, 17 are based on clinical factors, 19 incorporate clinical factors along with MRI details, 4 were based on blood biomarkers alone or in combination with clinical factors, and 5 included urinary biomarkers. The median AUC of externally validated RCs ranged from 0.63 to 0.93. This systematic review offers an extensive analysis of currently available RCs, their variable utilization, and performance within validation cohorts. RCs have consistently demonstrated their capacity to mitigate the limitations associated with early detection and have been integrated into modern practice and screening trials. Nevertheless, the lack of external validation data raises concerns about numerous RCs, and it is crucial to factor in this omission when evaluating whether a specific RC is applicable to one’s target population.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Prostate Cancer and Prostatic Diseases
医学-泌尿学与肾脏学
CiteScore
10.00
自引率
6.20%
发文量
142
审稿时长
6-12 weeks
期刊介绍:
Prostate Cancer and Prostatic Diseases covers all aspects of prostatic diseases, in particular prostate cancer, the subject of intensive basic and clinical research world-wide. The journal also reports on exciting new developments being made in diagnosis, surgery, radiotherapy, drug discovery and medical management.
Prostate Cancer and Prostatic Diseases is of interest to surgeons, oncologists and clinicians treating patients and to those involved in research into diseases of the prostate. The journal covers the three main areas - prostate cancer, male LUTS and prostatitis.
Prostate Cancer and Prostatic Diseases publishes original research articles, reviews, topical comment and critical appraisals of scientific meetings and the latest books. The journal also contains a calendar of forthcoming scientific meetings. The Editors and a distinguished Editorial Board ensure that submitted articles receive fast and efficient attention and are refereed to the highest possible scientific standard. A fast track system is available for topical articles of particular significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


