由二碘四炔中间体统一的烯二炔天然产物生物合成
IF 12.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
烯二炔类天然产物以其强大的细胞毒性而闻名于世,但其决定性的 1,5-二炔-3-烯核心分子的生物合成在很大程度上仍然是个谜。自 2002 年发现烯二炔多酮合成酶基因盒以来,基因组测序发现了数千个不同的烯二炔生物合成基因簇,每个基因簇都含有保守的烯二炔多酮合成酶基因盒。我们在此报告:(1) 该基因盒的产物是一个碘代庚炔、一个二碘四炔和两个戊炔;(2) 二碘四炔代表了所有已知烯二炔的共同生物合成中间体;(3) 可以利用隐性碘化来提高烯二炔的滴度。这些发现为烯二炔类化合物建立了统一的生物合成途径,为进一步推进烯二炔核心生物合成奠定了基础,并使烯二炔天然产物在化学、酶学和转化应用方面取得了根本性突破。本文章由计算机程序翻译,如有差异,请以英文原文为准。
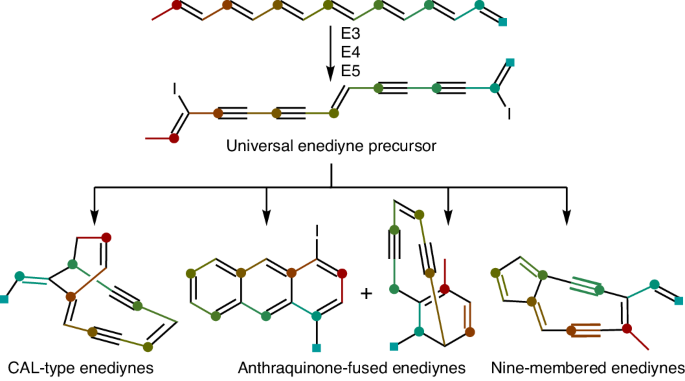
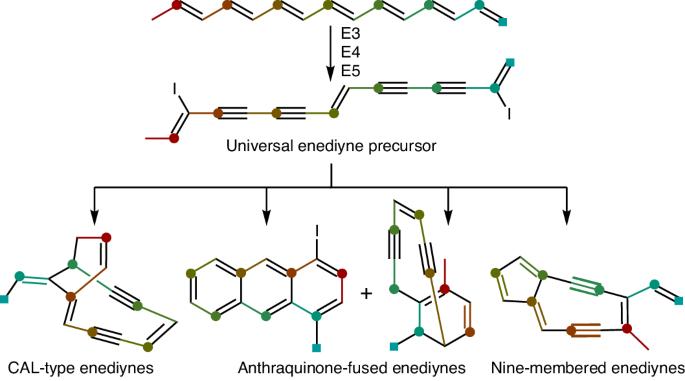
Enediyne natural product biosynthesis unified by a diiodotetrayne intermediate
Enediyne natural products are renowned for their potent cytotoxicities but the biosynthesis of their defining 1,5-diyne-3-ene core moiety remains largely enigmatic. Since the discovery of the enediyne polyketide synthase cassette in 2002, genome sequencing has revealed thousands of distinct enediyne biosynthetic gene clusters, each harboring the conserved enediyne polyketide synthase cassette. Here we report that (1) the products of this cassette are an iodoheptaene, a diiodotetrayne and two pentaynes; (2) the diiodotetrayne represents a common biosynthetic intermediate for all known enediynes; and (3) cryptic iodination can be exploited to increase enediyne titers. These findings establish a unified biosynthetic pathway for the enediynes, set the stage to further advance enediyne core biosynthesis and enable fundamental breakthroughs in chemistry, enzymology and translational applications of enediyne natural products. Enediyne natural products are potent antitumor antibiotics but the biosynthesis of their 1,5-diyne-3-ene core has remained enigmatic for decades. Here a diiodotetrayne is reported as a universal enediyne biosynthetic intermediate of this core, obtained upon cryptic iodination.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


