揭示癌症相关成纤维细胞在肿瘤转移中的关键作用:机制与治疗前景》。
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
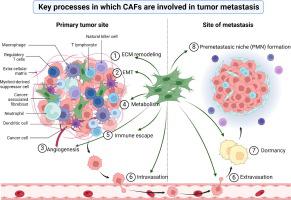
背景:肿瘤转移是一个循序渐进的过程,是癌症患者预后不良的主要决定因素。因此,深入探讨其机制具有重要的临床意义。癌症相关成纤维细胞(CAFs)是肿瘤微环境(TME)中最丰富的基质细胞群,其在肿瘤转移中的关键调控作用已获得有力的证据支持:1)细胞外基质(ECM)重塑;2)上皮-间质转化(EMT);3)血管生成;4)肿瘤代谢;5)血管周围迁移;6)免疫逃逸;7)休眠;8)转移前生态位(PMN)形成。此外,我们还提供了在癌症治疗中针对 CAFs 的现有策略简编。综述的关键科学概念 本综述为 CAFs 与肿瘤转移之间的相互作用勾勒了一个结构化框架,同时为潜在的治疗发展提供了见解。它有助于加深对肿瘤转移灶内癌症转移的理解,从而促进在抗转移疗法中利用CAF靶向疗法。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Unlocking the crucial role of cancer-associated fibroblasts in tumor metastasis: Mechanisms and therapeutic prospects
Background
Tumor metastasis represents a stepwise progression and stands as a principal determinant of unfavorable prognoses among cancer patients. Consequently, an in-depth exploration of its mechanisms holds paramount clinical significance. Cancer-associated fibroblasts (CAFs), constituting the most abundant stromal cell population within the tumor microenvironment (TME), have garnered robust evidence support for their pivotal regulatory roles in tumor metastasis.
Aim of review
This review systematically explores the roles of CAFs at eight critical stages of tumorigenic dissemination: 1) extracellular matrix (ECM) remodeling, 2) epithelial-mesenchymal transition (EMT), 3) angiogenesis, 4) tumor metabolism, 5) perivascular migration, 6) immune escape, 7) dormancy, and 8) premetastatic niche (PMN) formation. Additionally, we provide a compendium of extant strategies aimed at targeting CAFs in cancer therapy.
Key scientific concepts of review
This review delineates a structured framework for the interplay between CAFs and tumor metastasis while furnishing insights for the potential therapeutic developments. It contributes to a deeper understanding of cancer metastasis within the TME, facilitating the utilization of CAF-targeting therapies in anti-metastatic approaches.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


