Tfh 动态的转录调控与免疫突触的形成
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
在生殖中心(GC)内,抗原特异性B细胞依靠与免疫细胞的精确相互作用以及在暗区和亮区之间的战略定位来克隆扩增、亲和成熟并分化为长寿命浆细胞或记忆B细胞。滤泡辅助 T(Tfh)细胞是 GC 依赖性体液免疫的关键守门人,在次级淋巴组织内表现出显著的动态定位,在 GC 内分化和执行 B 细胞促进功能的过程中依赖与抗原提呈细胞(APC)的细胞间相互作用。在这篇综述中,我们简要介绍了 Tfh 细胞分化和功能的转录调控,并探讨了支配 Tfh 细胞运动的分子机制、它们与 GC 内 B 细胞的相互作用以及它们的动态行为对体液反应的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
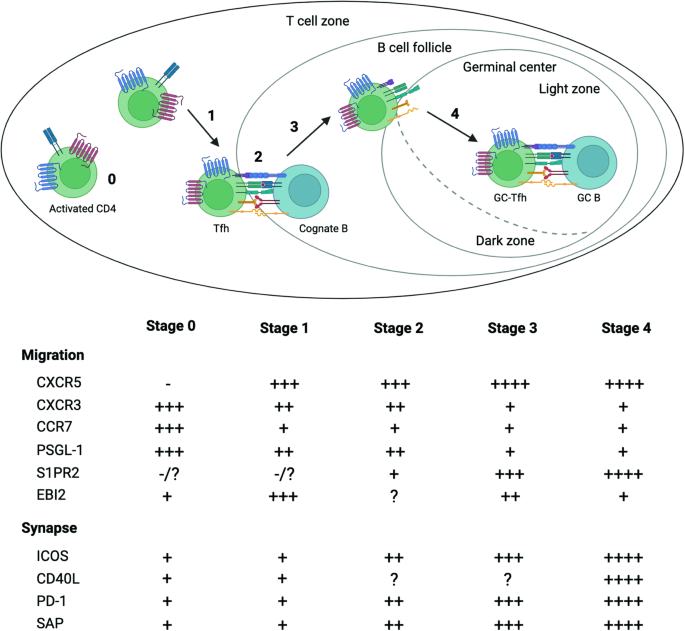
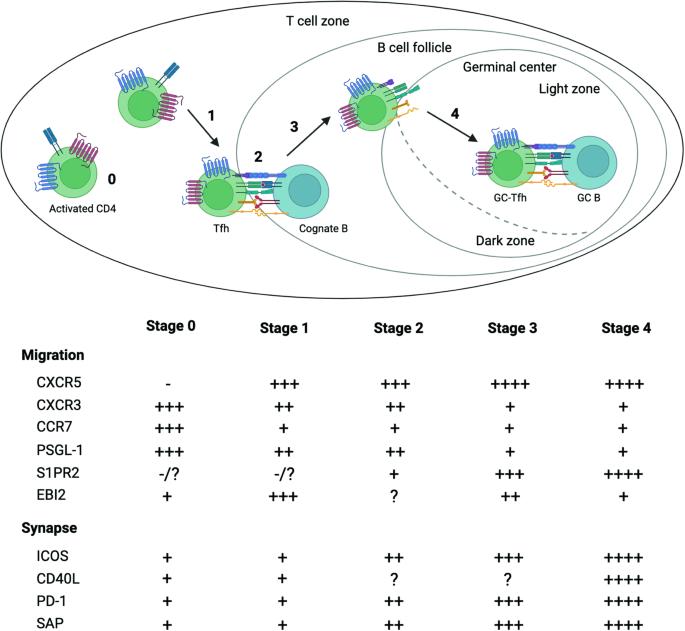
Transcriptional regulation of Tfh dynamics and the formation of immunological synapses
Inside germinal centers (GCs), antigen-specific B cells rely on precise interactions with immune cells and strategic localization between the dark and light zones to clonally expand, undergo affinity maturation, and differentiate into long-lived plasma cells or memory B cells. Follicular helper T (Tfh) cells, the key gatekeepers of GC-dependent humoral immunity, exhibit remarkable dynamic positioning within secondary lymphoid tissues and rely on intercellular interactions with antigen-presenting cells (APCs) during their differentiation and execution of B-cell-facilitating functions within GCs. In this review, we briefly cover the transcriptional regulation of Tfh cell differentiation and function and explore the molecular mechanisms governing Tfh cell motility, their interactions with B cells within GCs, and the impact of their dynamic behavior on humoral responses. This review focuses on T follicular helper (Tfh) cells—cells that are vital to antibody (Ab) production of the immune system. Led by Y.S. Choi, the team outlines how Tfh cells collaborate with B cells in our lymph nodes (small, bean-shaped glands throughout the body) to combat infections. The authors delve into genetic and molecular processes dictating Tfh cells’ actions. Key takeaways highlight the need for precise positioning and timing of Tfh cells with B cells, as well as the critical role of Tfh cell-B cell interaction intensity in B cell affinity maturation. The authors conclude the article by emphasizing the importance of gaining insights into these interactions to develop new therapeutic strategies for autoimmune diseases (conditions where the immune system mistakenly attacks the body), and to enhance vaccine responses. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


