胃基质微环境对胃移行细胞和发育不良的调控。
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
对与胃癌发生相关的微环境的研究主要集中在胃癌上,但往往低估了癌前病变阶段,如移行期和发育不良期。由于上皮细胞与 T 细胞、巨噬细胞和 2 型先天性淋巴细胞(ILC2)的相互作用是胃癌前病变形成不可或缺的因素,因此了解促进胃癌前病变的细胞相互作用值得进一步研究。虽然各种类型的免疫细胞已被证明在胃癌发生中发挥重要作用,但成纤维细胞等基质细胞如何影响胃上皮转化,尤其是在癌前病变阶段,目前仍不清楚。成纤维细胞作为不同的群体存在于不同的组织中,并根据细胞表面标志物和分泌因子的表达模式发挥不同的功能。在这篇综述中,我们概述了基质中已知的微环境成分,重点是成纤维细胞亚群及其在乳腺、胰腺和胃等组织癌变过程中的作用。此外,我们还深入探讨了肿瘤促进成纤维细胞的潜在靶点,并确定了与成纤维细胞可塑性和胃癌发生调控相关的开放研究领域。本文章由计算机程序翻译,如有差异,请以英文原文为准。
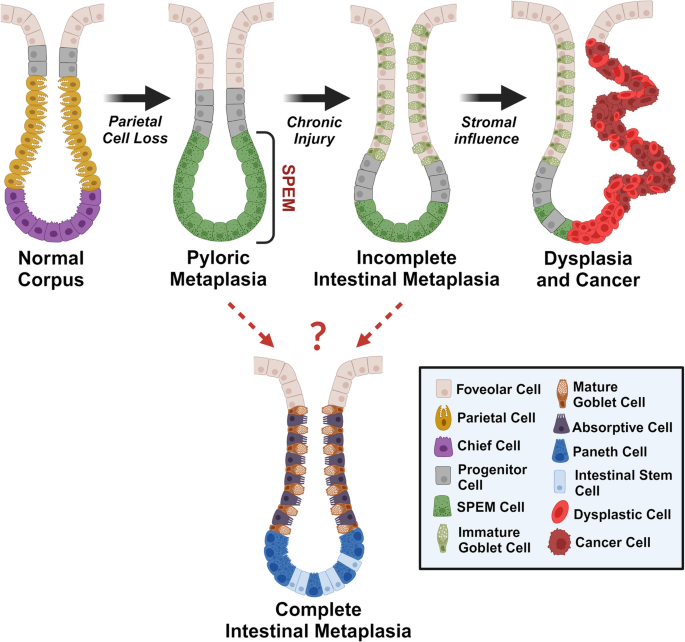
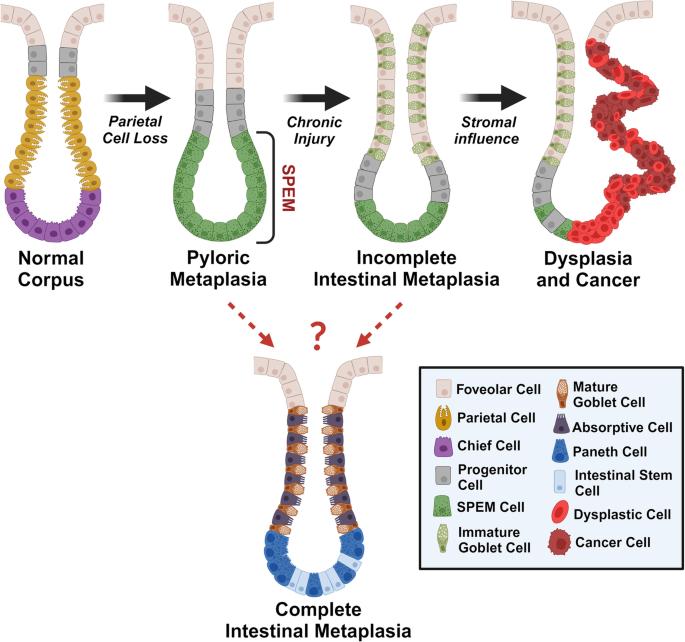
Regulation of metaplasia and dysplasia in the stomach by the stromal microenvironment
Research on the microenvironment associated with gastric carcinogenesis has focused on cancers of the stomach and often underestimates premalignant stages such as metaplasia and dysplasia. Since epithelial interactions with T cells, macrophages, and type 2 innate lymphoid cells (ILC2s) are indispensable for the formation of precancerous lesions in the stomach, understanding the cellular interactions that promote gastric precancer warrants further investigation. Although various types of immune cells have been shown to play important roles in gastric carcinogenesis, it remains unclear how stromal cells such as fibroblasts influence epithelial transformation in the stomach, especially during precancerous stages. Fibroblasts exist as distinct populations across tissues and perform different functions depending on the expression patterns of cell surface markers and secreted factors. In this review, we provide an overview of known microenvironmental components in the stroma with an emphasis on fibroblast subpopulations and their roles during carcinogenesis in tissues including breast, pancreas, and stomach. Additionally, we offer insights into potential targets of tumor-promoting fibroblasts and identify open areas of research related to fibroblast plasticity and the modulation of gastric carcinogenesis. This review summarizes how metaplasia (normal cells changing into different types) turns into dysplasia (abnormal cell growth) in the stomach due to injury and the role of stroma during the process. Led by Dr. James R. Goldenring, the team has discovered that the stomach develops metaplasia to heal damaged tissue and suggested several biomarkers to define the change. They recently found that certain types of metaplasia repopulate the stomach lining after damage and can progress into the next stage under the influence of the microenvironment. The current study provides insights into how the stromal components in the stomach contribute to carcinogenesis, focusing on fibroblast subpopulations. This could be important for future gastric cancer treatments. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


