环硅酸锆钠治疗高频肾衰竭和高钾血症:REALIZE-K 设计和基线特征
IF 10.3
1区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
摘要
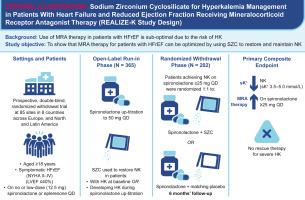
矿物皮质激素受体拮抗剂(MRAs)可改善射血分数降低型心力衰竭(HFrEF)患者的预后。然而,由于存在高钾血症问题,MRA 通常未得到充分利用。为了评估环硅酸锆钠(SZC)这种能捕获并快速降低血钾的非吸收晶体是否能让高钾血症(或高风险)患者使用 MRA。REALIZE-K 是一项前瞻性、双盲、安慰剂对照试验,适用于 HFrEF(NYHA 功能分级 II/IV;左心室射血分数≤40%)、接受过最佳治疗(MRA 除外)和高钾血症流行(或高风险)的患者。在开放标签试运行期间,所有参与者都接受了方案规定的螺内酯滴定(目标值:每天 50 毫克);在滴定过程中出现高钾血症(队列 1)或偶发高钾血症(队列 2)的参与者开始服用 SZC。每天服用螺内酯≥25毫克时达到正常血钾的参与者被随机分配到继续服用SZC或匹配的安慰剂,为期6个月。主要的复合终点是获得最佳应答(正常血钾、每天服用螺内酯≥25毫克、高血钾无需抢救[1-6个月])的参与者比例。在 365 名患者(试验期)中,有 202 人接受了随机治疗。基线特征包括平均年龄 70 岁、普遍的合并症(78% 的估计肾小球滤过率<60 mL/min/1.73 m,37% 的心房颤动)、高 N 末端前 B 型钠尿肽(中位数 1,136 pg/mL)和高 HFrEF 治疗(64% 使用沙库比曲利/缬沙坦,96% 使用β-受体阻滞剂,42% 使用钠葡萄糖协同转运体 2 抑制剂)。在随机化时,75% 的患者每天服用 50 毫克螺内酯。REALIZE-K 是首个评估 SZC 是否能快速、安全地优化 MRA 并使高钾血症流行/高危患者长期服药的试验。(评估 SZC 对接受螺内酯治疗的症状性 HFrEF 患者高钾血症管理的有效性和安全性的研究;)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Sodium Zirconium Cyclosilicate in HFrEF and Hyperkalemia
Background
Mineralocorticoid receptor antagonists (MRAs) improve outcomes in patients with heart failure and reduced ejection fraction (HFrEF). However, MRAs are often underused because of hyperkalemia concerns.
Objectives
The purpose of this study was to assess whether sodium zirconium cyclosilicate (SZC), a nonabsorbed crystal that traps and rapidly lowers potassium, enables MRA use in patients with HFrEF and prevalent hyperkalemia (or at high risk).
Methods
REALIZE-K is a prospective, double-blind, placebo-controlled trial in patients with HFrEF (NYHA functional class II-IV; left ventricular ejection fraction ≤40%), optimal therapy (except MRA), and prevalent hyperkalemia (or at high risk). During the open-label run-in, all participants underwent protocol-mandated spironolactone titration (target: 50 mg daily); those with prevalent (cohort 1) or incident (cohort 2) hyperkalemia during titration started SZC. Participants achieving normokalemia while on spironolactone ≥25 mg daily were randomized to continuing SZC or matching placebo for 6 months. The primary composite endpoint was proportion of participants with optimal response (normokalemia, on spironolactone ≥25 mg daily, no rescue for hyperkalemia [months 1-6]).
Results
Of 365 patients (run-in), 202 were randomized. Baseline characteristics included mean age 70 years, prevalent comorbidities (78% estimated glomerular filtration rate <60 mL/min/1.73 m2, 38% atrial fibrillation/flutter), high N-terminal pro B-type natriuretic peptide (median 1,136 pg/mL), and high HFrEF therapy use (64% sacubitril/valsartan, 96% beta-blocker, 42% sodium glucose co-transporter 2 inhibitor). At randomization, 78% were receiving spironolactone 50 mg daily.
Conclusions
REALIZE-K is the first trial to evaluate whether SZC can enable rapid and safe MRA optimization and long-term continuation in patients with HFrEF and prevalent/high risk of hyperkalemia. (Study to Assess Efficacy and Safety of SZC for the Management of High Potassium in Patients with Symptomatic HFrEF Receiving Spironolactone [REALIZE-K]; NCT04676646)
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

JACC. Heart failure
CARDIAC & CARDIOVASCULAR SYSTEMS-
CiteScore
21.20
自引率
2.30%
发文量
164
期刊介绍:
JACC: Heart Failure publishes crucial findings on the pathophysiology, diagnosis, treatment, and care of heart failure patients. The goal is to enhance understanding through timely scientific communication on disease, clinical trials, outcomes, and therapeutic advances. The Journal fosters interdisciplinary connections with neuroscience, pulmonary medicine, nephrology, electrophysiology, and surgery related to heart failure. It also covers articles on pharmacogenetics, biomarkers, and metabolomics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


