m6A 阅读器 YTHDF2 在调节免疫逃避中的肿瘤内在作用。
IF 17.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
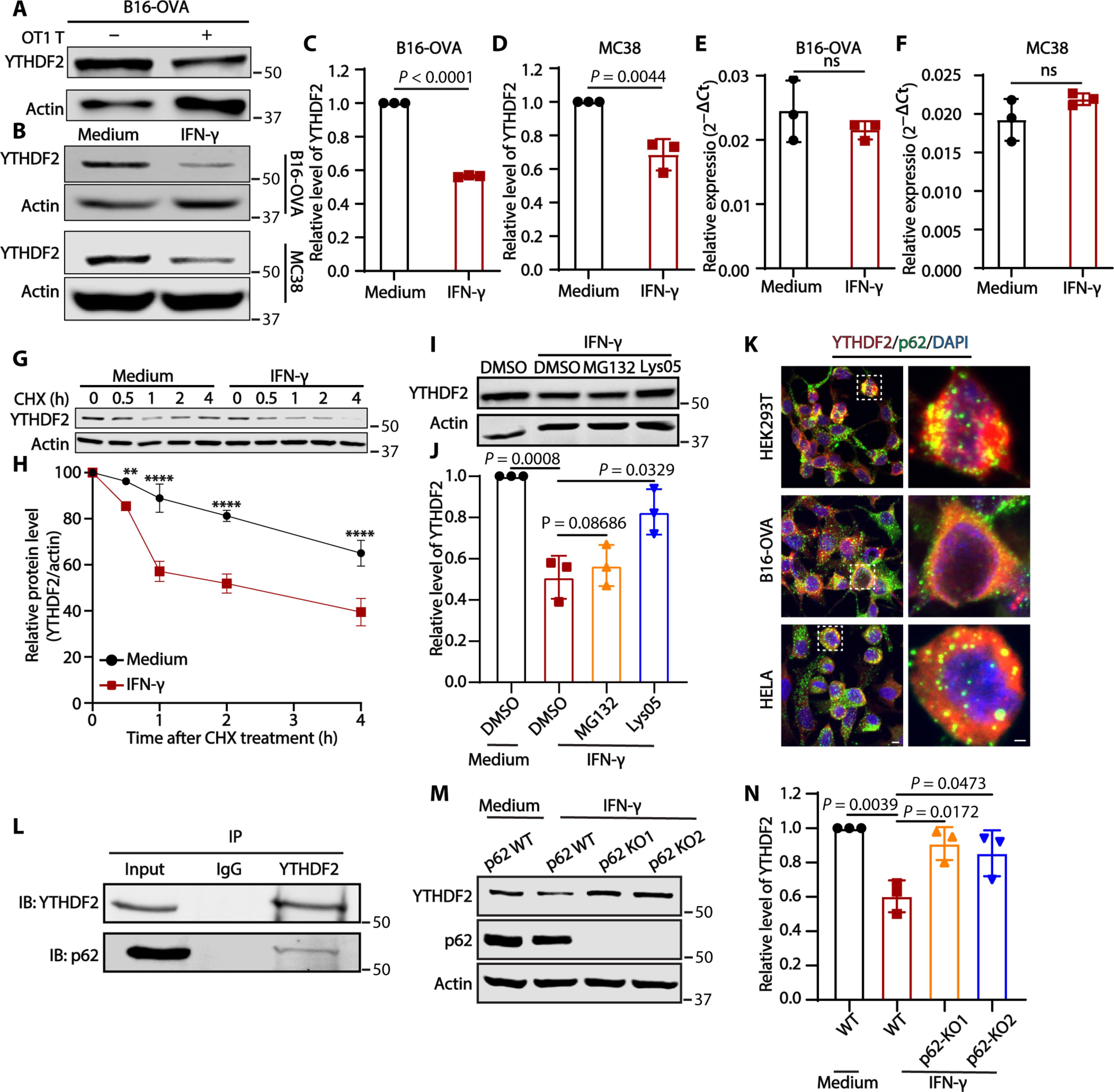
肿瘤通过各种机制逃避免疫系统的攻击。在这里,我们发现了由含YTH结构域的家族蛋白2(YTHDF2)介导的肿瘤免疫逃避的一个组成部分,YTHDF2是一种阅读蛋白,通常会破坏m6A修饰的mRNA的稳定性。在免疫功能正常的肿瘤模型中,肿瘤 YTHDF2 的缺失可抑制肿瘤生长并延长存活时间。从机理上讲,肿瘤 YTHDF2 缺乏会通过 CX3CL1 促进巨噬细胞的招募,并通过损害肿瘤糖酵解代谢增强 CD8+ T 细胞的线粒体呼吸。在有 IFN-γ 的情况下,肿瘤 YTHDF2 缺乏会促进炎性巨噬细胞极化和抗原递呈。此外,IFN-γ 还能诱导肿瘤 YTHDF2 自噬降解,从而使肿瘤细胞对 CD8+ T 细胞介导的细胞毒性敏感。最后,我们发现了一种能优先诱导YTHDF2降解的小分子化合物,这种化合物单独使用时具有强效抗肿瘤作用,但与抗PD-L1或抗PD-1抗体联合使用时效果更好。总之,YTHDF2似乎是一种协调免疫逃避的肿瘤内在调控因子,是增强癌症免疫疗法的一个有希望的靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The tumor-intrinsic role of the m6A reader YTHDF2 in regulating immune evasion
Tumors evade attacks from the immune system through various mechanisms. Here, we identify a component of tumor immune evasion mediated by YTH domain–containing family protein 2 (YTHDF2), a reader protein that usually destabilizes m6A-modified mRNA. Loss of tumoral YTHDF2 inhibits tumor growth and prolongs survival in immunocompetent tumor models. Mechanistically, tumoral YTHDF2 deficiency promotes the recruitment of macrophages via CX3CL1 and enhances mitochondrial respiration of CD8+ T cells by impairing tumor glycolysis metabolism. Tumoral YTHDF2 deficiency promotes inflammatory macrophage polarization and antigen presentation in the presence of IFN-γ. In addition, IFN-γ induces autophagic degradation of tumoral YTHDF2, thereby sensitizing tumor cells to CD8+ T cell–mediated cytotoxicity. Last, we identified a small molecule compound that preferentially induces YTHDF2 degradation, which shows a potent antitumor effect alone but a better effect when combined with anti–PD-L1 or anti–PD-1 antibodies. Collectively, YTHDF2 appears to be a tumor-intrinsic regulator that orchestrates immune evasion, representing a promising target for enhancing cancer immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Immunology
Immunology and Microbiology-Immunology
CiteScore
32.90
自引率
2.00%
发文量
183
期刊介绍:
Science Immunology is a peer-reviewed journal that publishes original research articles in the field of immunology. The journal encourages the submission of research findings from all areas of immunology, including studies on innate and adaptive immunity, immune cell development and differentiation, immunogenomics, systems immunology, structural immunology, antigen presentation, immunometabolism, and mucosal immunology. Additionally, the journal covers research on immune contributions to health and disease, such as host defense, inflammation, cancer immunology, autoimmunity, allergy, transplantation, and immunodeficiency. Science Immunology maintains the same high-quality standard as other journals in the Science family and aims to facilitate understanding of the immune system by showcasing innovative advances in immunology research from all organisms and model systems, including humans.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


