热力学上不受欢迎的取代六元环的合成技术
IF 38.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
六元环是生物活性化合物和多功能材料中无处不在的结构主题。值得注意的是,六元环的热力学不赞成异构体,如一个取代基位于赤道位置、另一个位于轴向位置的二取代环己烷,与它们的相反异构体相比,往往具有更强的物理和生物活性。然而,热力学上不被看好的异构体的合成本质上具有挑战性,可能的方法数量有限。在本综述中,我们总结并比较了产生热力学不利取代模式的取代六元环的合成方法。我们特别强调阐明每种转化中的关键立体诱导因素。我们的目的是激发人们对合成这些独特结构的兴趣,同时为合成化学家提供应对这一合成挑战的指南。本文章由计算机程序翻译,如有差异,请以英文原文为准。
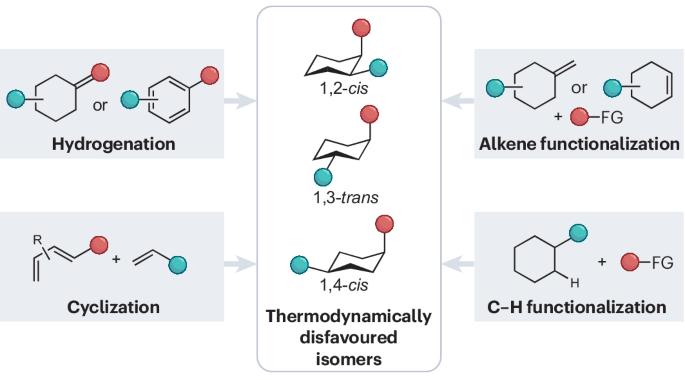
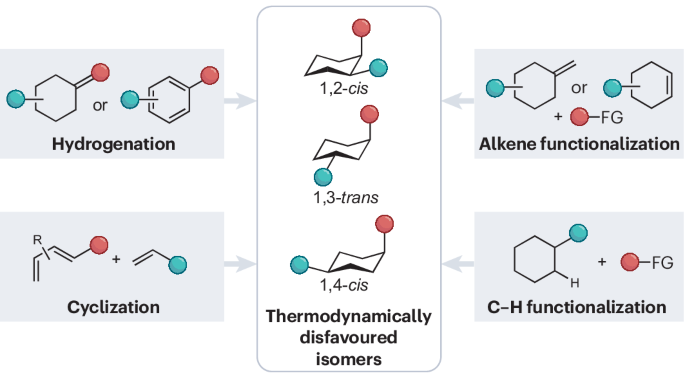
Synthetic techniques for thermodynamically disfavoured substituted six-membered rings
Six-membered rings are ubiquitous structural motifs in bioactive compounds and multifunctional materials. Notably, their thermodynamically disfavoured isomers, like disubstituted cyclohexanes featuring one substituent in an equatorial position and the other in an axial position, often exhibit enhanced physical and biological activities in comparison with their opposite isomers. However, the synthesis of thermodynamically disfavoured isomers is, by its nature, challenging, with only a limited number of possible approaches. In this Review, we summarize and compare synthetic methodologies that produce substituted six-membered rings with thermodynamically disfavoured substitution patterns. We place particular emphasis on elucidating the crucial stereoinduction factors within each transformation. Our aim is to stimulate interest in the synthesis of these unique structures, while simultaneously providing synthetic chemists with a guide to approaching this synthetic challenge. The synthesis of thermodynamically disfavoured substituted six-membered rings provides a notable challenge compared with that of the thermodynamically stable stereoisomer counterparts. This Review provides a summary of current strategies for their synthesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature reviews. Chemistry
Chemical Engineering-General Chemical Engineering
CiteScore
52.80
自引率
0.80%
发文量
88
期刊介绍:
Nature Reviews Chemistry is an online-only journal that publishes Reviews, Perspectives, and Comments on various disciplines within chemistry. The Reviews aim to offer balanced and objective analyses of selected topics, providing clear descriptions of relevant scientific literature. The content is designed to be accessible to recent graduates in any chemistry-related discipline while also offering insights for principal investigators and industry-based research scientists. Additionally, Reviews should provide the authors' perspectives on future directions and opinions regarding the major challenges faced by researchers in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


