整合全基因组 CRISPR 筛选和硅学药物分析,开发靶向解毒剂。
IF 13.1
1区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
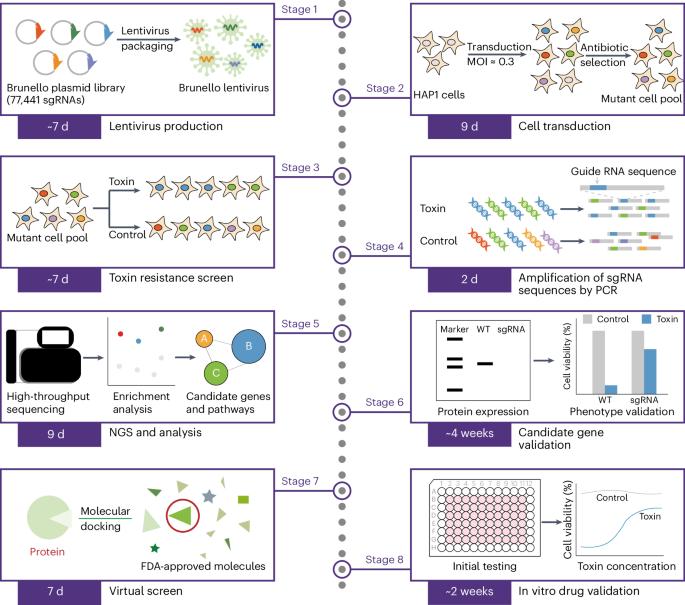
威胁人类的毒素不计其数,但大多数毒素都没有特效解毒剂。尽管 CRISPR 筛选有助于发现一些毒素的机制,但开发有针对性的解毒剂仍是一项重大挑战。最近,我们建立了一个开发解毒剂的系统框架,将利用全基因组CRISPR筛选鉴定新型药物靶点与美国食品药品管理局批准的药物虚拟筛选相结合。这种方法可以在全基因组水平上全面了解毒素机制,并有助于确定针对特定分子的有前景的解毒药物。在这里,我们将逐步说明如何在 HAP1 细胞中执行基因组规模的 CRISPR-Cas9 毒素敲除筛选。我们还提供了进行硅学药物筛选和体内药物验证的详细指导。使用该方案,进行基因组规模筛选需要约 4 周时间,测序和数据分析需要 4 周时间,验证候选基因需要 4 周时间,虚拟筛选需要 1 周时间,体外药物验证需要 2 周时间。这一框架有可能加快多种毒素解毒剂的开发,并能迅速确定已知安全有效的有前途的候选药物。这样就能比传统方法更快地开发出新的解毒剂,保护生命免受各种毒素的伤害,促进人类健康。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Integrating genome-wide CRISPR screens and in silico drug profiling for targeted antidote development
Numerous toxins threaten humans, but specific antidotes are unavailable for most of them. Although CRISPR screening has aided the discovery of the mechanisms of some toxins, developing targeted antidotes remains a significant challenge. Recently, we established a systematic framework to develop antidotes by combining the identification of novel drug targets by using a genome-wide CRISPR screen with a virtual screen of drugs approved by the US Food and Drug Administration. This approach allows for a comprehensive understanding of toxin mechanisms at the whole-genome level and facilitates the identification of promising antidote drugs targeting specific molecules. Here, we present step-by-step instructions for executing genome-scale CRISPR–Cas9 knockout screens of toxins in HAP1 cells. We also provide detailed guidance for conducting an in silico drug screen and an in vivo drug validation. By using this protocol, it takes ~4 weeks to perform the genome-scale screen, 4 weeks for sequencing and data analysis, 4 weeks to validate candidate genes, 1 week for the virtual screen and 2 weeks for in vitro drug validation. This framework has the potential to accelerate the development of antidotes for a wide range of toxins and can rapidly identify promising drug candidates that are already known to be safe and effective. This could lead to the development of new antidotes much more quickly than traditional methods, protecting lives from diverse toxins and advancing human health. This protocol integrates genome-wide CRISPR knockout screening of toxins with in silico drug profiling for a new approach to targeted antidote development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Protocols
生物-生化研究方法
CiteScore
29.10
自引率
0.70%
发文量
128
审稿时长
4 months
期刊介绍:
Nature Protocols focuses on publishing protocols used to address significant biological and biomedical science research questions, including methods grounded in physics and chemistry with practical applications to biological problems. The journal caters to a primary audience of research scientists and, as such, exclusively publishes protocols with research applications. Protocols primarily aimed at influencing patient management and treatment decisions are not featured.
The specific techniques covered encompass a wide range, including but not limited to: Biochemistry, Cell biology, Cell culture, Chemical modification, Computational biology, Developmental biology, Epigenomics, Genetic analysis, Genetic modification, Genomics, Imaging, Immunology, Isolation, purification, and separation, Lipidomics, Metabolomics, Microbiology, Model organisms, Nanotechnology, Neuroscience, Nucleic-acid-based molecular biology, Pharmacology, Plant biology, Protein analysis, Proteomics, Spectroscopy, Structural biology, Synthetic chemistry, Tissue culture, Toxicology, and Virology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


