10 型成骨不全症和常见免疫疾病的细胞支架。
IF 4.5
3区 医学
Q1 GENETICS & HEREDITY
引用次数: 0
摘要
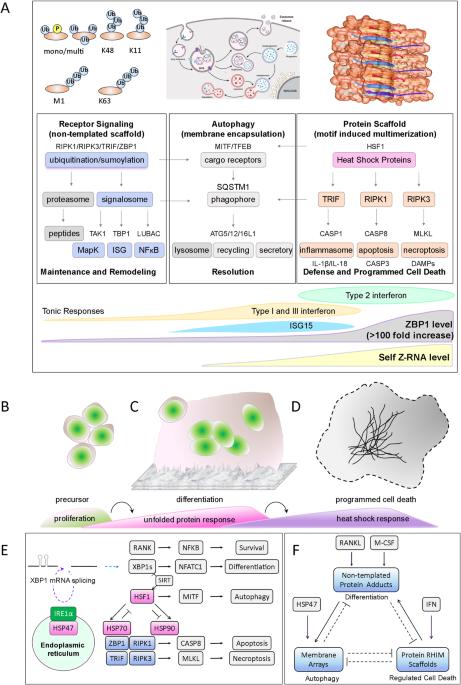
10 型成骨不全症(OI10)是由编码热休克蛋白 47(HSP47)的基因 SERPINH1 的功能缺失密码子变异引起的,而不是由指定骨形成的基因引起的。HSP47 变体会破坏胶原蛋白和内切酶 IRE1α(肌醇需要酶 1α)的折叠,而 IRE1α能剪接 X-Box 结合蛋白 1(XBP1)mRNA。除了影响骨骼发育外,变体还可能影响破骨细胞的分化。三种不同的生化支架在破骨细胞的分化和调控细胞死亡过程中发挥着关键作用。这些支架由非模板蛋白修饰、有序脂质阵列和蛋白丝组成。这些支架成分是由基因指定的,但会在细胞外扰动因子、病原体和翻转子基因组编码的左旋 Z-RNA 螺旋的作用下组装起来。其结果取决于 RIPK1、RIPK3、TRIF 和 ZBP1 之间通过称为 RHIMs 的短相互作用基序进行的相互作用。导致 HSP47 的非同义替换发生在与 RHIMs 关系密切的新型变异亮氨酸重复区域(vLRR)中。其他 vLRR 蛋白变异也是多种不同泯灭性疾病的诱因。驱动 "亡羊补牢 "病理学的支架也与许多其他复杂疾病的结果有关。它们的组装是由flipons和其他特定语境开关动态触发的,而不是由 "亡羊补牢 "式的因果密码子变异触发的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Osteogenesis imperfecta type 10 and the cellular scaffolds underlying common immunological diseases
Osteogenesis imperfecta type 10 (OI10) is caused by loss of function codon variants in the gene SERPINH1 that encodes heat shock protein 47 (HSP47), rather than in a gene specifying bone formation. The HSP47 variants disrupt the folding of both collagen and the endonuclease IRE1α (inositol-requiring enzyme 1α) that splices X-Box Binding Protein 1 (XBP1) mRNA. Besides impairing bone development, variants likely affect osteoclast differentiation. Three distinct biochemical scaffold play key roles in the differentiation and regulated cell death of osteoclasts. These scaffolds consist of non-templated protein modifications, ordered lipid arrays, and protein filaments. The scaffold components are specified genetically, but assemble in response to extracellular perturbagens, pathogens, and left-handed Z-RNA helices encoded genomically by flipons. The outcomes depend on interactions between RIPK1, RIPK3, TRIF, and ZBP1 through short interaction motifs called RHIMs. The causal HSP47 nonsynonymous substitutions occur in a novel variant leucine repeat region (vLRR) that are distantly related to RHIMs. Other vLRR protein variants are causal for a variety of different mendelian diseases. The same scaffolds that drive mendelian pathology are associated with many other complex disease outcomes. Their assembly is triggered dynamically by flipons and other context-specific switches rather than by causal, mendelian, codon variants.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Genes and immunity
医学-免疫学
CiteScore
8.90
自引率
4.00%
发文量
28
审稿时长
6-12 weeks
期刊介绍:
Genes & Immunity emphasizes studies investigating how genetic, genomic and functional variations affect immune cells and the immune system, and associated processes in the regulation of health and disease. It further highlights articles on the transcriptional and posttranslational control of gene products involved in signaling pathways regulating immune cells, and protective and destructive immune responses.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


