利用可食用多酚姜黄素进行光诱导糖基化。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在可见光照射(470 纳米)条件下,研究人员考察了使用一种可食用多酚姜黄素对三氯乙酰亚氨酸供体和醇进行光诱导糖基化的情况。研究首次发现,这些糖基化反应在温和的反应条件下顺利进行,并以高产率得到相应的糖苷。此外,本糖基化方法适用于多种三氯乙酰亚氨酸供体和醇受体,并对亚磷酸糖基、磷酸盐、(N-苯基)三氟乙酰亚氨酸、氟化物、糖醛和硫代糖苷显示出很高的化学选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
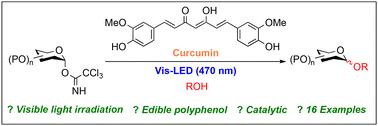
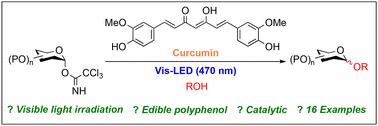
Photo-induced glycosylation using the edible polyphenol curcumin†
Photo-induced glycosylations of trichloroacetimidate donors and alcohols using an edible polyphenol, curcumin, were examined under visible photo-irradiation (470 nm). It was found, for the first time, that these glycosylations proceed smoothly under mild reaction conditions to give the corresponding glycosides in high yields. In addition, the present glycosylation method was applicable to a wide range of trichloroacetimidate donors and alcohol acceptors and showed high chemoselectivity over glycosyl phosphite, phosphate, (N-phenyl)trifluoroacetimidate, fluoride, glycal and thioglycoside.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


