MYC 相分离选择性地调节转录组
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
包括 MYC 和 MYCN 在内的 MYC 转录因子(TFs)的失调和表达增强是导致大多数人类癌症的原因。例如,在高危神经母细胞瘤中,MYCN 的扩增可高达几百倍。由此导致的 N-myc 过度表达会异常激活 N-myc 水平较低时未被激活的基因,并推动细胞增殖。N-myc水平的升高是仅仅介导了与基因组中亲和力较低的结合位点的结合,还是从根本上改变了激活过程,目前仍不清楚。N-myc水平超过阈值后可能变得重要的一种激活机制是通过相分离形成异常转录凝聚物。相分离最近被认为与转录调控有关,但它在多大程度上有助于基因激活仍是一个未决问题。在这里,我们对 N-myc 的相行为进行了表征,结果表明它可以形成具有转录特征的动态凝聚体。我们利用化学遗传学工具测试了相分离在 N-myc 调控转录中的作用,该工具允许我们在同等 N-myc 水平下比较非相分离条件和相分离条件,与无 N-myc 表达相比,这两种条件对基因表达都有很大影响。有趣的是,我们发现只有一小部分(<3%)N-myc调控基因受到相分离的进一步调控,但这些事件包括关键致癌基因的激活和肿瘤抑制因子的抑制。事实上,相分离增加了细胞增殖,证实了转录变化的生物学效应。然而,我们的研究结果还表明,97%的N-myc调控基因不受N-myc相分离的影响,这表明TFs与转录机制的可溶性复合物足以激活转录。本文章由计算机程序翻译,如有差异,请以英文原文为准。
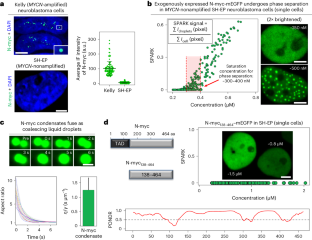
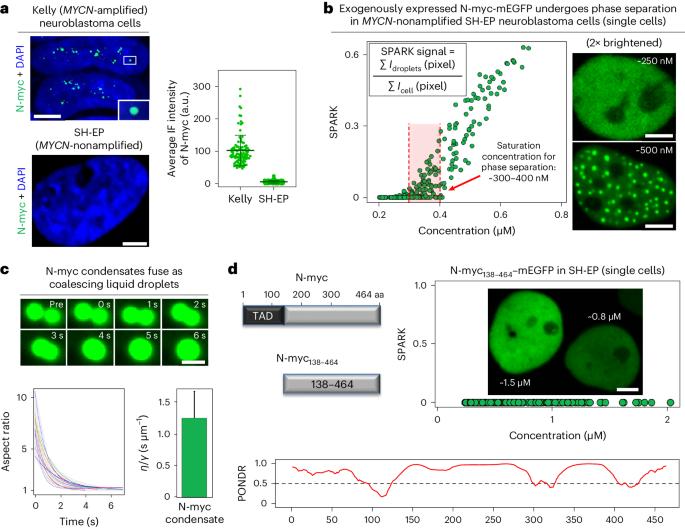
MYC phase separation selectively modulates the transcriptome
Dysregulation and enhanced expression of MYC transcription factors (TFs) including MYC and MYCN contribute to the majority of human cancers. For example, MYCN is amplified up to several hundredfold in high-risk neuroblastoma. The resulting overexpression of N-myc aberrantly activates genes that are not activated at low N-myc levels and drives cell proliferation. Whether increasing N-myc levels simply mediates binding to lower-affinity binding sites in the genome or fundamentally changes the activation process remains unclear. One such activation mechanism that could become important above threshold levels of N-myc is the formation of aberrant transcriptional condensates through phase separation. Phase separation has recently been linked to transcriptional regulation, but the extent to which it contributes to gene activation remains an open question. Here we characterized the phase behavior of N-myc and showed that it can form dynamic condensates that have transcriptional hallmarks. We tested the role of phase separation in N-myc-regulated transcription by using a chemogenetic tool that allowed us to compare non-phase-separated and phase-separated conditions at equivalent N-myc levels, both of which showed a strong impact on gene expression compared to no N-myc expression. Interestingly, we discovered that only a small percentage (<3%) of N-myc-regulated genes is further modulated by phase separation but that these events include the activation of key oncogenes and the repression of tumor suppressors. Indeed, phase separation increases cell proliferation, corroborating the biological effects of the transcriptional changes. However, our results also show that >97% of N-myc-regulated genes are not affected by N-myc phase separation, demonstrating that soluble complexes of TFs with the transcriptional machinery are sufficient to activate transcription. Oncoprotein transcription factor MYC undergoes phase separation, forming transcriptionally active condensates. The chemogenetic tool SPARK-ON reveals that MYC phase separation selectively modulates the transcriptome and promotes cell proliferation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


