通过有机催化实现两个 C(sp3)-H 键的电驱动对映选择性交叉脱氢偶联
IF 8.9
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本研究报告采用无金属催化剂,甚至无需额外的氧化剂,就能实现两个 C(sp3)-H 键的交叉脱氢偶联反应 (CDC) 的高效、可扩展的电化学不对称方案。研究表明,一系列醛类(包括天然产物)和含有 C(sp3)-H 键的各种底物(包括呫吨、吖啶、环庚三烯甚至二芳基甲烷)都能发生不对称 CDC 反应,生成一系列碳-碳键偶联产物,产率高达 94%,ee 值高达 98%。机理研究(如自由基时钟实验)表明,该反应是在电化学条件下通过烯胺的亲核攻击进行的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
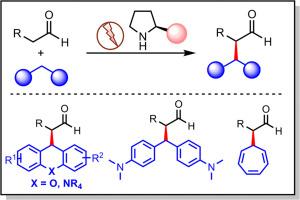
Electricity-driven enantioselective cross-dehydrogenative coupling of two C(sp3)-H bonds enabled by organocatalysis
An efficient and scalable electrochemical asymmetric protocol with metal-free catalysts and even without additional oxidants for the cross-dehydrogenative coupling reaction (CDC) of two C(sp3)-H bonds is reported. A series of aldehydes including natural products and various substrates containing C(sp3)-H bonds including xanthenes, acridines, cycloheptatrienes and even diarylmethane have been shown to undergo asymmetric CDC to afford a series of carbon-carbon bond coupling products with up to 94% yield and 98% ee. Mechanistic studies such as radical clock experiment suggest that the reaction proceeds via nucleophilic attack by enamine under electrochemical conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


