在交联 DNA 上停滞的 XPD 可深入了解损伤验证情况
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
超家族 2 螺旋酶 XPD 是一般转录因子 II H(TFIIH)的核心成分,它对转录和核苷酸切割 DNA 修复(NER)至关重要。在这两个过程中,XPD 的螺旋酶功能对 NER 至关重要,但对转录起始并不重要,在转录起始过程中,XPD 只充当其他因子的支架。我们利用低温电子显微镜破译了 XPD 螺旋酶作用中最神秘的步骤之一:主动分离双链 DNA(dsDNA),并在接近 DNA 链间交联(一种剧毒的 DNA 损伤形式)时停滞。该结构显示了dsDNA是如何分离的,并揭示了Arch结构域在主动分离dsDNA过程中极不寻常的参与。结合诱变和生化分析,我们确定了对螺旋酶活性非常重要的不同功能区。令人惊讶的是,这些区域也影响了 TFIIH 核心转位酶的活性,揭示了 XPD 在 TFIIH 支架中尚未遇到的功能。总之,我们的数据为 NER 气泡形成、XPD 损伤验证和 XPG 切割提供了一个普遍基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
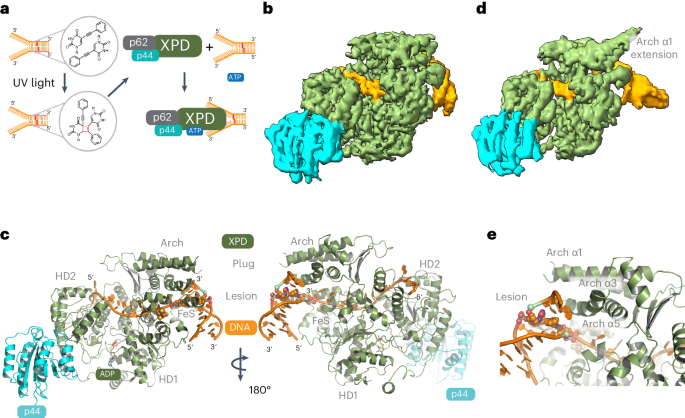
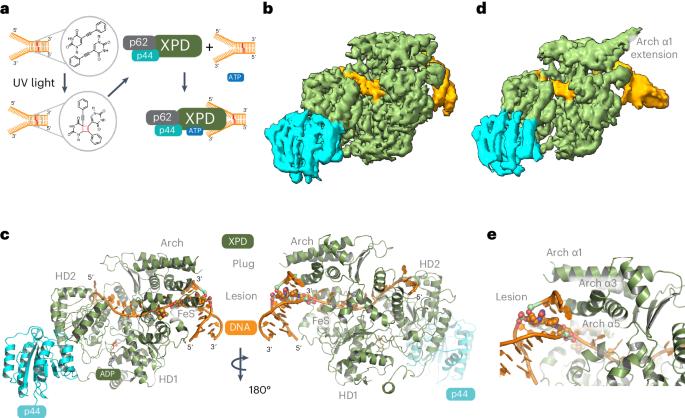
XPD stalled on cross-linked DNA provides insight into damage verification
The superfamily 2 helicase XPD is a central component of the general transcription factor II H (TFIIH), which is essential for transcription and nucleotide excision DNA repair (NER). Within these two processes, the helicase function of XPD is vital for NER but not for transcription initiation, where XPD acts only as a scaffold for other factors. Using cryo-EM, we deciphered one of the most enigmatic steps in XPD helicase action: the active separation of double-stranded DNA (dsDNA) and its stalling upon approaching a DNA interstrand cross-link, a highly toxic form of DNA damage. The structure shows how dsDNA is separated and reveals a highly unusual involvement of the Arch domain in active dsDNA separation. Combined with mutagenesis and biochemical analyses, we identified distinct functional regions important for helicase activity. Surprisingly, those areas also affect core TFIIH translocase activity, revealing a yet unencountered function of XPD within the TFIIH scaffold. In summary, our data provide a universal basis for NER bubble formation, XPD damage verification and XPG incision. Here, using cryo-EM and biochemistry, the authors delineate how the XPD helicase unorthodoxly uses its Arch domain to separate double-stranded DNA upon approaching a DNA lesion, promoting our understanding of NER bubble formation and damage verification.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


