金(I)催化的(E)-酮-N,O-乙醛环化:螺-噁唑-γ-内酯的合成路线。
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在这项研究中,我们利用均相金(I)催化技术开发了一种内酮-N,O-乙醛的级联 5,5 环化反应。这一过程包括最初的 5-外-二元碳环化,然后是 5-外-二元杂环化,立体选择性地将水分子的 O 原子结合到 N-系丙炔中。这种顺序反应形成了一个 C-C、两个 C-O 和两个 C-I 键,最终以良好的收率合成了具有噁唑环的螺-α-碘-γ-内酯结构。本文章由计算机程序翻译,如有差异,请以英文原文为准。
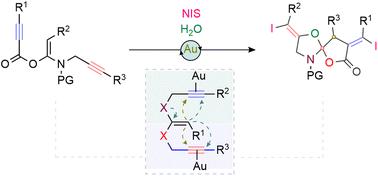
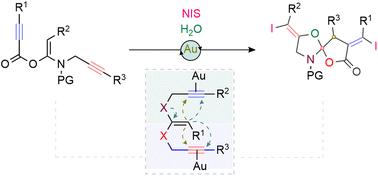
Gold(i)-catalysed cyclisation of (E)-ketene-N,O-acetals: a synthetic route toward spiro-oxazole-γ-lactones†‡
In this study, we developed a cascade 5,5-cyclisation of internal ketene-N,O-acetals utilizing homogeneous Au(i) catalysis. This process involves an initial 5-exo-dig carbocyclisation, followed by a 5-exo-dig heterocyclisation that stereoselectively incorporates the O-atom of a water molecule into an N-tethered propargyl alkyne. This sequential reaction results in the formation of one C–C, two C–O, and two C–I bonds, ultimately leading to the synthesis of spiro-α-iodo-γ-lactone structures featuring oxazole rings in good yields.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
The international home of synthetic, physical and biomolecular organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


