在实际环境中使用英克利西兰进行降脂治疗:来自全国医疗服务机构的初步数据
IF 3.6
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
背景clisiran是一种小干扰RNA,可长期抑制9型碱性磷酸酶(PCSK9)的合成,在临床试验中表现出良好的安全性和有效性。目前还缺乏有关实现血脂目标和减少治疗差距的潜力的真实世界数据。方法来自一家全国性医疗机构的数据,涉及 2022 年 3 月至 2023 年 11 月期间开始使用 inclisiran 的患者。结果503 名患者(57% 为女性;平均年龄为 66±11 岁)开始使用 inclisiran。54%的患者患有心血管疾病,64%的患者LDL-C峰值为190 mg/dL。28%的患者曾接触过PCSK9单克隆抗体。397例患者(347例注射次数≥2次)在首次处方后2个月获得了血脂谱。仅接受 inclisiran 治疗的患者(n = 254)中,LDL-C 从峰值水平降低的中位数为 57%(四分位距 [IQR],48%-67%),从注射前水平降低的中位数为 40%(19%-54%)。在同时接受降脂治疗的患者中(n = 143),LDL-C 从峰值水平降低的中位数为 66%(IQR,55%-73%),从注射前水平降低的中位数为 46%(23%-59%)。39%的患者 LDL-C < 70 mg/dL,21.9%的患者 LDL-C < 55 mg/dL。在同时接受他汀类药物治疗的患者中,38%达到了 LDL-C < 55 mg/dL。总的来说,6.5% 的患者在首次注射后中断了 inclisiran 治疗。然而,由于联合降脂疗法的使用有限,且我们的患者队列中严重高胆固醇血症的比例较高,因此血脂目标的达标率并不理想。本文章由计算机程序翻译,如有差异,请以英文原文为准。
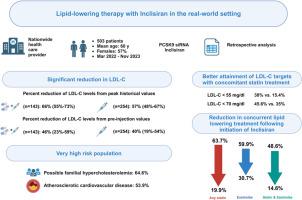
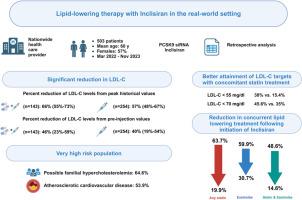
Lipid-lowering therapy with inclisiran in the real-world setting: Initial data from a national health care service
BACKGROUND
Inclisiran, a small-interfering RNA enabling long-term inhibition of proprotein convertase subtilisin kexin type 9 (PCSK9) synthesis, demonstrates a good safety and efficacy profile in clinical trials. Real-world data on the potential to attain lipid-goals and reduce treatment gaps are lacking.
OBJECTIVES
To investigate the implementation of inclisiran in real-world clinical setting.
METHODS
Data from a nationwide healthcare organization on patients initiating inclisiran between 3/2022–11/2023. Patients’ characteristics, lipid-lowering therapies, post-treatment reduction in low-density lipoprotein cholesterol (LDL-C), and attainment of treatment goals were evaluated.
RESULTS
Inclisiran was initiated by 503 patients (57% women; mean age 66±11 years). Cardiovascular disease was present in 54%, and peak LDL-C levels >190 mg/dL documented in 64%. Prior exposure to PCSK9 monoclonal antibodies was evident in 28%. Lipid profile >2 months after filling first prescription, was available in 397 patients (347 with ≥2 injections). In patients treated by inclisiran only (n = 254), median LDL-C reduction from peak levels was 57% (interquartile range [IQR], 48%-67%), and from pre-injection levels 40% (19%-54%). In those with concomitant lipid-lowering therapies (n = 143), median LDL-C reduction from peak levels was 66% (IQR, 55%-73%), and from pre-injection levels 46% (23%-59%). LDL-C < 70 mg/dL was attained by 39% and LDL-C < 55 mg/dL by 21.9%. Of those treated with concomitant statin therapy, 38% attained LDL-C < 55 mg/dL. Overall, 6.5% discontinued inclisiran therapy after initial injection.
CONCLUSIONS
In real-world practice, inclisiran showed good efficacy in reducing LDL-C with high interindividual variability. However, attainment rates of lipid goals were suboptimal due to limited use of combination lipid-lowering therapy and high rates of severe hypercholesterolemia in our patient population cohort.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.00
自引率
6.80%
发文量
209
审稿时长
49 days
期刊介绍:
Because the scope of clinical lipidology is broad, the topics addressed by the Journal are equally diverse. Typical articles explore lipidology as it is practiced in the treatment setting, recent developments in pharmacological research, reports of treatment and trials, case studies, the impact of lifestyle modification, and similar academic material of interest to the practitioner.
Sections of Journal of clinical lipidology will address pioneering studies and the clinicians who conduct them, case studies, ethical standards and conduct, professional guidance such as ATP and NCEP, editorial commentary, letters from readers, National Lipid Association (NLA) news and upcoming event information, as well as abstracts from the NLA annual scientific sessions and the scientific forums held by its chapters, when appropriate.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


