活动性溃疡性结肠炎 fmt 的严格供体选择:从一项因徒劳无益而停止的随机对照试验中汲取的关键教训。
IF 11.6
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
背景和目的:根据微生物群水平对供体进行严格的预选、严格的厌氧处理以及反复使用 FMT,假设可提高 FMT 诱导 UC 缓解的效果:RESTORE-UC试验是一项多中心、双盲、假对照、随机试验。中重度 UC 患者(定义为总马约 4-10)被随机分配接受四次厌氧制备的异体或自体供体 FMT。异基因供体材料是根据微生物细胞数、肠型和特定菌属的丰度进行严格筛选后选出的。主要终点是第 8 周时的无类固醇临床缓解(梅奥总分≤2,无子分值>1)。在66%(n=72)的预定纳入患者(n=108)后,进行了预先计划的无效性分析。在第 0 周和第 8 周进行了微生物组定量分析(n=44):共纳入 72 名患者,其中 66 人至少接受了一次 FMT(异基因 FMT 30 人,自体 FMT 36 人)。在第 8 周,分别有 3 名和 5 名患者达到了无类固醇临床缓解的主要终点(P=0.72),这表明异基因-FMT 的治疗差异不超过 5%。因此,该研究因无效而停止。微生物组分析显示,与自体骨髓移植相比,异基因骨髓移植的肠型转换在数量上更多,而且当患者接受的肠型与基线时的肠型不同时,观察到的肠型转换也更多(p=0.01)。原发反应与基线时较低的梅奥总评分、较低的细菌细胞计数和较高的 Bacteroides 2 感染率有关:RESTORE-UC试验并未达到其主要终点,即在第8周时无类固醇临床缓解率有所提高。进一步的研究还应考虑患者选择、灭菌假对照、增加频率、密度以及在用药前进行 FMT 的可行性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
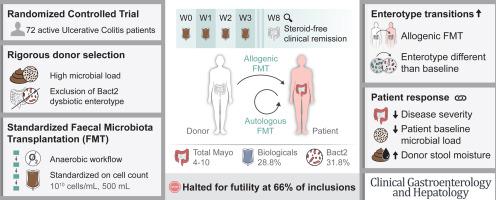
Rigorous Donor Selection for Fecal Microbiota Transplantation in Active Ulcerative Colitis: Key Lessons From a Randomized Controlled Trial Halted for Futility
Background & Aims
Rigorous donor preselection on microbiota level, strict anaerobic processing, and repeated fecal microbiota transplantation (FMT) administration were hypothesized to improve FMT induction of remission in ulcerative colitis (UC).
Methods
The RESTORE-UC trial was a multi-centric, double-blind, sham-controlled, randomized trial. Patients with moderate to severe UC (defined by total Mayo 4–10) were randomly allocated to receive 4 anaerobic-prepared allogenic or autologous donor FMTs. Allogenic donor material was selected after a rigorous screening based on microbial cell count, enterotype, and the abundance of specific genera. The primary endpoint was steroid-free clinical remission (total Mayo ≤2, no sub-score >1) at week 8. A pre-planned futility analysis was performed after 66% (n = 72) of intended inclusions (n = 108). Quantitative microbiome profiling (n = 44) was performed at weeks 0 and 8.
Results
In total, 72 patients were included, of which 66 received at least 1 FMT (allogenic FMT, n = 30 and autologous FMT, n = 36). At week 8, respectively, 3 and 5 patients reached the primary endpoint of steroid-free clinical remission (P = .72), indicating no treatment difference of at least 5% in favor of allogenic FMT. Hence, the study was stopped due to futility. Microbiome analysis showed numerically more enterotype transitions upon allogenic FMT compared with autologous FMT, and more transitions were observed when patients were treated with a different enterotype than their own at baseline (P = .01). Primary response was associated with lower total Mayo scores, lower bacterial cell counts, and higher Bacteroides 2 prevalence at baseline.
Conclusion
The RESTORE-UC trial did not meet its primary endpoint of increased steroid-free clinical remission at week 8. Further research should additionally consider patient selection, sterilized sham-control, increased frequency, density, and viability of FMT prior to administration.
ClinicalTrials.gov, Number: NCT03110289.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
16.90
自引率
4.80%
发文量
903
审稿时长
22 days
期刊介绍:
Clinical Gastroenterology and Hepatology (CGH) is dedicated to offering readers a comprehensive exploration of themes in clinical gastroenterology and hepatology. Encompassing diagnostic, endoscopic, interventional, and therapeutic advances, the journal covers areas such as cancer, inflammatory diseases, functional gastrointestinal disorders, nutrition, absorption, and secretion.
As a peer-reviewed publication, CGH features original articles and scholarly reviews, ensuring immediate relevance to the practice of gastroenterology and hepatology. Beyond peer-reviewed content, the journal includes invited key reviews and articles on endoscopy/practice-based technology, health-care policy, and practice management. Multimedia elements, including images, video abstracts, and podcasts, enhance the reader's experience. CGH remains actively engaged with its audience through updates and commentary shared via platforms such as Facebook and Twitter.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


