调节非水环境中的水氢键,控制其在电化学转化中的反应活性
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
电化学二氧化碳还原(CO2R)可以为生产燃料和化学品提供一条可持续发展的途径;然而,二氧化碳还原的选择性经常会受到相互竞争的氢进化反应(HER)的影响,即使在水的浓度很小的情况下也是如此。在这里,我们调整了一系列具有不同官能团和理化性质的钝化溶剂中水的溶解度和动力学,以调节 HER 活性和 CO2R 选择性。我们的研究表明,通过将水限制在强氢键网络中,可以将 HER 的起始电位提高近 1 V。然后,我们利用金催化剂在水浓度高达 3 M 的条件下实现了近 100% 的 CO 法拉第效率。此外,在弱酸性条件下,我们使用富土锌催化剂在长期电解过程中保持了近 100% 的 CO 法拉第效率,且无碳酸盐损失。我们的工作为控制水的反应性提供了见解,并揭示了指导重要电化学转化的电解质设计的描述符。本文章由计算机程序翻译,如有差异,请以英文原文为准。
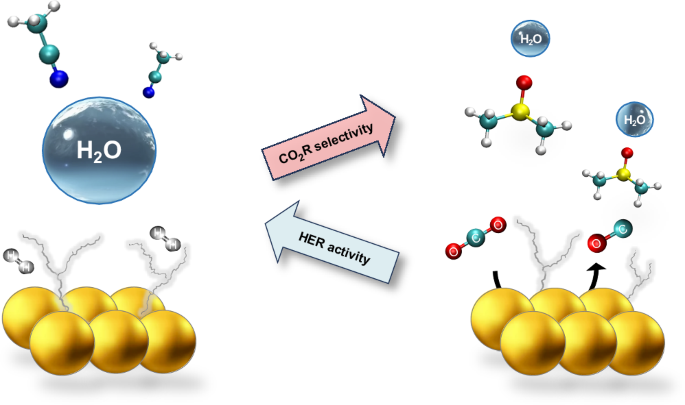
Modulating water hydrogen bonding within a non-aqueous environment controls its reactivity in electrochemical transformations
Electrochemical carbon dioxide reduction (CO2R) can provide a sustainable route to produce fuels and chemicals; however, CO2R selectivity is frequently impaired by the competing hydrogen evolution reaction (HER), even for small concentrations of water. Here we tune water solvation and dynamics within a series of aprotic solvents featuring different functional groups and physicochemical properties to modulate HER activity and CO2R selectivity. We show that one can extend the HER onset potential by almost 1 V by confining water within a strong hydrogen bond network. We then achieve nearly 100% CO Faradaic efficiency at water concentrations as high as 3 M with a gold catalyst. Furthermore, under mildly acidic conditions, we sustain nearly 100% Faradaic efficiency towards CO with no carbonate losses over long-term electrolysis with an earth-abundant zinc catalyst. Our work provides insights to control water’s reactivity and reveals descriptors to guide electrolyte design for important electrochemical transformations. Electroreduction of CO2 competes with the hydrogen evolution reaction; thus, controlling water’s activity to exclusively act as a proton donor is a desirable yet challenging goal. Now the behaviour of water in aprotic solvents is shown to depend on the solvent’s donor ability, which can modulate the hydrogen bond network and in turn promote the desired reactivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


